-
Category
-
ActivityInsecticide
-
PremixPyrethrins+Azadirachtin
PIPERONYL BUTOXIDE+PYRETHRINS
Concentrate in oil and water, usually containing synergists; in impregnated and stabilized dust concentrates; in dilute dusts made from ground flowers. In recent years a low-color, 20% pyrethrin extract in oil has become the "standard" item of the industry, although less concentrated solutions in oil are still available.
Premix Parters: carbosulfan; imidacloprid; sulfur;
-
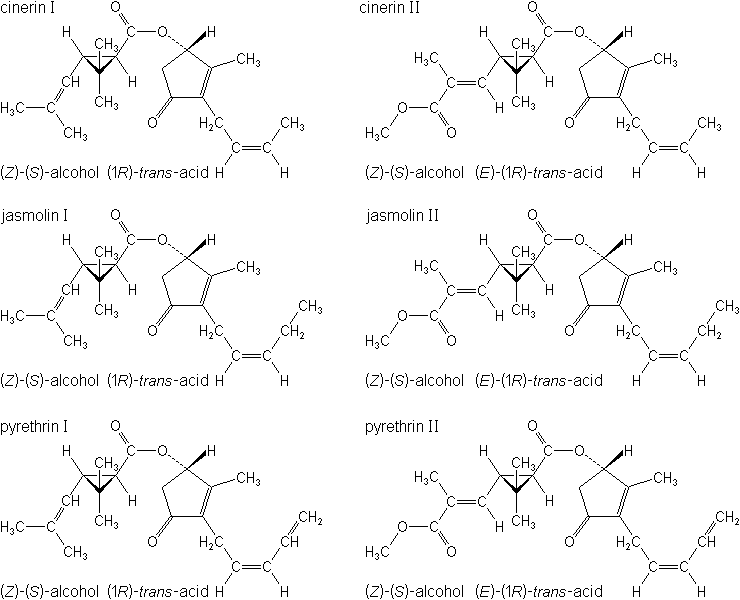
Physical PropertiesBotanical insecticide whose active principles are pyrethrins I and II (esters of pyrethrolone and chrysanthemic acid and pyrethroic acid), cinerins I and II (esters of cinerolone and chrysanthemic and pyrethroic acids), and jasmolin I and II (jasmoline and chrysanthemic and pyrethroic acids), collectively known as the "pyrethrins." The plant Chrysanthemum cinerariaefolium, and the flowers are the source of the principles. The flowers and extracts are principally imported from Kenya, Rwanda, Tanzania, and Ecuador. Pyrethrum dried flowers contain 0.9-1.3% pyrethrins. The crude extract or oleoresin contains 50-60% pyrethrins and most refined grade (pale) about 50% (20% in U.S.) pyrethrins by dewaxing and decoloring. Formerly the dried flowers were known as Dalmatian insect flowers; the powdered flowers as "insect powder.".
-
ToxicologyOral:Acute oral LD50 for male rats 2370, female rats 1030, mice 273-796 mg/ kg. Percutaneous:Acute percutaneous LD50 for rats >1500, rabbits 5000 mg/kg. Slightly irritating to skin and eyes. Constituents of the flowers may cause dermatitis to sensitised individuals, but are removed during the preparati Inhalation: LC50 (4 h) for rats 3.4 mg/l.
-
Environmental ProfileEcotoxicology:
Bees:Toxic to bees, but exhibits a repellent effect. LD50 (oral) 22 ng/bee; (contact) 130-290 ng/bee.Birds:Acute oral LD50 for mallard ducks >10 000 mg/ kg.Daphnia: LC50 12µg/l.Fish:Highly toxic to fish. LC50 (96 h) (static tests) for coho salmon 39, channel catfish 114 mg/l. LC50 for bluegill sunfish 10, rainbow trout 5.2µg/l.
Environmental fate:
Animals:In mammals, rapidly degraded in the stomach by hydrolysis of the ester bond to harmless metabolites. For details, see T. J. Class et al., J. Agric. Food Chem., 1990, 38, 529.Soil:In the environment, degradation, promoted by sunlight and u.v. light, begins at the alcohol group and involves the formation of numerous unknown cleavage products. WATER SOLUBILITY: Soluble -
Transport InformationSignal Word:CAUTION; Hazard Class:II(Moderately hazardous)
Porduct NewsMore
Bayer develops alternative to glyphosate herbicide
Colombia’s Constitutional Court bans chlorpyrifos
ADAMA Canada moves forward with lambda-cyhalothrin sales for 2023
Mexico: Import of glyphosate drops from 16,500 tons to 8,200 tons
Glyphosate prices drop 30% in Argentina
One year extension of EU glyphosate approval failed to pass in the latest meeting
Palmer amaranth in two southern U.S. states now resistant to S-metolachlor
Related CompaniesMore
Beijing Kingbo Biotech Co.,Ltd
Country: China
Pyrethrins Oxymatrine Rotenone Nicotine Physcion Berberine Extract of reynoutria sachalinensis Osthol Matrine Carvacrol

 0
0 Subscribe
Subscribe