-
Common NameCadusafos
-
中文通用名硫线磷
-
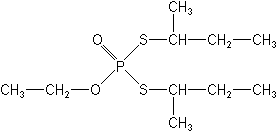
IUPACS,S-di-sec-butyl O-ethyl phosphorodithioate
-
CASO-ethyl S,S-bis(1-methylpropyl) phosphorodithioate
-
CAS No.95465-99-9
-
Molecular FormulaC10H23O2PS2
-
Molecular Structure
-
Category
-
ActivityInsecticide.
Cadusafos is a contact and stomach insecticide/nematicide. It is applied to the soil: either to the furrow at the time of planting, or to the soil surface. It is not absorbed by roots, but has been shown to enhance root development. The product is not systemic, but low soil mobility ensures that the product is in contact with the root system for a protracted period after application.
In field trials, cadusafos gave equal or better control of nematodes and wireworms than standard insecticides/nematicides, sometimes at lower use rates. -
CropUseCrop uses:
bananas, citrus, coffee, fruits, maize, peanuts, potatoes, soybeans, sugar beets, sugar cane, tobacco, vegetables, vines, watermelons
Maize
750-2,000 g ai/ha
Soybeans
1,000-2,000 g ai/ha
Sugar cane
2,000-4,000 g ai/ha
Vines
5-15 g ai/ha
Bananas
2-3 g ai/plant per application
Peanuts
1,000-2,000 g ai/ha
Vegetables
2,000-9,000 g ai/ha
Tobacco
3,000-6,000 g ai/ha
-
Physical PropertiesMolecular weight:270.4; Physical form:Colourless to yellow liquid. Density:1.054 (20 °C); Flash point:129.4 °C (Seta closed cup); Vapour pressure:1.2 ×102 mPa (25 °C); Partition coefficient(n-octanol and water):logP = 3.9; Solubility:In water 248 mg/l. Completely miscible with acetone, acetonitrile, dichloromethane, ethyl acetate, toluene, methanol, isopropanol, and heptane.; Stability:Stable up to 50 °C. Half-life in light <115 d.
-
ToxicologyOral:Acute oral LD50 for rats 37.1, mice 71.4 mg/kg. Percutaneous:Acute percutaneous LD50 for male rabbits 24.4, female rabbits 41.8 mg/kg. Non-irritating to skin and practically non-irritating to eyes of rabbits. Inhalation:LC50 (4 h) for rats 0.026 mg/l air.?
-
Environmental ProfileEcotoxicology: Algae:EC50 (96 h) 5.3 mg/l.Birds:Acute oral LD50 for bobwhite quail 16, mallard ducks 230 mg/kg.Daphnia:LC50(48 h) 1.6
mg/l.Fish:LC50(96 h) for rainbow trout 0.13, bluegill sunfish 0.17 mg/l.Worms:LC50(14 d) for Eisenia foetida 72 mg/kg. Environmental fate: Animals:Readily absorbed, metabolised and eliminated in urine and faeces. Hydroxy sulfones were the major metabolites, followed by phosphorothioic and sulfonic acids.Soil:DT50 in silty clay, sandy loam soils 11-55 d. Koc 144-351.
Mallard duck
LD50 230 mg/kg
Bobwhite quail
LD50 16.4 mg/kg
Rainbow trout [96 h]
LC50 130 μg/L
Fate in soil:
Cadusafos is slightly mobile in sandy soils. Its soil half-life is approximately 6-7 weeks. The product does not bioaccumulate from season to season.Fate in aquatic systems:
Cadusafos undergoes hydrolysis at pH 5. Its half-life in water is 4-5 weeks.
WATER SOLUBILITY: 248 ppm?
SOIL PARTICLE ADSORPTION: Half-life of 45 days in silty clay, sandy loam soils. -
Transport InformationSignal Word:WARNING; Hazard Class:Ib (Highly hazardous)
Porduct NewsMore
Bayer develops alternative to glyphosate herbicide
Colombia’s Constitutional Court bans chlorpyrifos
ADAMA Canada moves forward with lambda-cyhalothrin sales for 2023
Mexico: Import of glyphosate drops from 16,500 tons to 8,200 tons
Glyphosate prices drop 30% in Argentina
One year extension of EU glyphosate approval failed to pass in the latest meeting
Palmer amaranth in two southern U.S. states now resistant to S-metolachlor

 0
0 Subscribe
Subscribe