-
Common NameCinidon-ethyl
-
中文通用名吲哚酮草酯
-
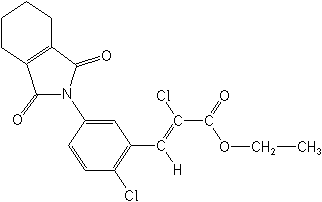
IUPACethyl (Z)-2-chloro-3-[2-chloro-5-(cyclohex-1-ene-1,2-dicarboximido)phenyl]acrylate
-
CASethyl (2Z)-2-chloro-3-[2-chloro-5-(1,3,4,5,6,7-hexahydro-1,3-dioxo-2H-isoindol-2-yl)phenyl]-2-propenoate
-
CAS No.142891-20-1
-
Molecular FormulaC19H17Cl2NO4
-
Molecular Structure
-
Category
-
ActivityHerbicide.
Cinidon-ethyl is a contact herbicide. Due to its high lipophilicity, it is rapidly absorbed by the plant cuticle following application and effects can be seen within 48 hours. The product is most effective on smaller weeds up to the 4-5 leaf stage, and on Galium aparine up to 4 to 5 whorls. The treated weeds are usually dead within three weeks of application. The product can be applied from the two true leaf stage through to before the third node stage for winter wheat and durum, and to before the first node for winter rye. The product is rainfast one hour after application and its activity is not affected by low temperatures, allowing it to be used early in the growing season.
Mode of action studies concluded that cinidon-ethyl is a protox-inhibiting, peroxidizing herbicide which has contact activity in the green tissue of sensitive weed species. No significant differences have been observed between foliar and root absorption or translocation of the herbicide by Solanumnigrum, Galiumaparine and wheat [Pesticide Science, 1999]. Foliar or root application of [14C]-cinidon-ethyl revealed that there was minimal translocation of the parent herbicide to untreated parts of the plant. The tolerance of wheat to cinidon-ethyl is suggested to be the consequence of a more rapid metabolism, coupled with moderate leaf absorption. This tolerance results in a selectivity index (SI) of approximately 500 between wheat and S nigrum or G aparine (SI = IC30 (wheat)/IC30 (weed)).
In radiolabel studies, treated leaves (species as above) were found to contain cinidon-ethyl and its initial metabolites, the E-isomer of the parent (BAS 615M00) and the acid (BAS 615M01). When applied to plants post-emergence, these metabolites demonstrated approximately 10-fold lower herbicidal activity than the parent compound.
Field trials carried out in Germany (BCPC, 1999) with winter wheat and barley compared cinidon-ethyl with fluroxpyr and amidosulfuron and found it to be equal or superior at controlling Galium aparine with a faster speed of control.
The product is ideal for combinations with foliar and soil residual herbicides, increasing the speed of activity and reducing the dependency on weather conditions of the combination partners. BASF recommends tank-mixes with mecoprop-P (Duplosan) and betazone + dichlorprop-P (Basagran DP) to extend cinidon-ethyl’s activity to volunteer oilseed rape (Brassica napus) and chickweed (Stellaria media) and to control larger cleavers. In field trials conducted in France, 30 - 50 g ai/ha of cinidon-ethyl tank mixed with isoproturon plus diflufenican was found to improve the post-emergence autumn weed control of Galium aparine, Papaver rhoeas and Veronica spp.
BASF has recorded some transient necrotic spotting on crops. The company reports that this does not affect the yield of the crop. -
CropUseCrop uses:
barley, rye, triticale, wheat
Cereals
50 g ai/ha
-
Premix
Emulsifiable concentrate. Premix Parters: piperophos.Type
AI concn
Emulsifiable concentrate (EC)
20% (w/v)
-
Physical PropertiesMolecular weight:394.26; Physical form:White, crystalline, odourless powder. Density:1.398 (20 °C); Composition:Tech. is >90%. Melting point:112.2-112.7 °C; Vapour pressure:<1×10-2 mPa (20 °C); Henry constant:<6.91×10-2 Pa m3 mol-1; Partition coefficient(n-octanol and water):logP = 4.51 (25 °C); Solubility:In water 0.057 mg/l (20 °C). In acetone 213, methanol 8, toluene 384 (all in g/l, 20 °C). Stability:Rapidly hydrolysed and photolysed. Hydrolysis DT50 5 d (pH 5), 35 h (pH 7), 54 min(pH 9) (20 °C). Photolysis DT50 2.3 d.
-
ToxicologyOral:Acute oral LD50 for rats >2200 mg/kg. Percutaneous:Acute percutaneous LD50 for rats >2000 mg/kg. Not a skin or eye irritant (rabbits). A skin sensitiser (guinea pigs). Inhalation:LC50 (4 h) for rats >5.3 mg/l.?
-
Environmental ProfileEcotoxicology: Algae:EbC50 0.02 mg/l; NOEC 21.9 mg/l.Bees:LD50 (oral and contact) >200
mg/bee.Birds:Acute oral LD50 for bobwhite quail >2000 mg/kg.Daphnia:LC50 52.1 mg/l.Fish:LC50 (96 h) for rainbow trout >24.8 mg/l.Worms:LC50 >1000 mg/kg soil. Environmental fate: Readily biodegradable in soil and water.Animals:Following limited, but rapid, absorption, and widespread distribution in organs and tissues, the a.i. is extensively metabolised and rapidly excreted.Soil:Soil DT50 0.6-2 d (lab., aerobic conditions, 20 °C); rapidly mineralised. Rapidly degraded in water; decomposition increases with increasing alkalinity. Not stable photolytically in water.Plant:The a.i. is extensively metabolised; no bioavailable components of the residues present in grain are likely to be found at toxicologically significant levels.
WATER SOLUBILITY: 57 μg/l at 20°C.
Bobwhite quail
LD50 >2,000 mg/kg
Rainbow trout
LC50 >200 mg/L
Algae
EbC50 = 0.021 mg/L
Lemna gibba
EbC50 = 0.174 mg/L
Daphnia[48 h]
LC50 59 mg/L
Bee [48 h, oral and contact]
LD50 >200 μg/bee
Earthworm [14 d]
LC50 >1000 mg/kg soil
Fate in soil:
Cinidon-ethyl is non-mobile in soil. It has a half-life of less than four days. There is a minimal risk of leaching into ground water.
In laboratory studies under aerobic soil conditions at 20°C, the DT50 was found to vary between 0.6 and 1.9 days (mean value of 1.3), and the DT90 between 6 and 22 days (mean value of 15). The three major metabolites 615M01, 615M03 and 615M04 were found to have a DT50 of 10-54 days, 23-33 days and 13 days at 20°C, respectively. In anaerobic soil at 20°C, the DT50 was 0.3 days for cinidon-ethyl whilst 615M01 had a DT50 of 14 days and 615M03 of 18 days. Field studies were not required.
The Koc value for cinidon-ethyl was found to be 869 to 5654 in four soil types. The metabolite 615M01 had a Koc of 90 to 435 and 615M03 from 0 to >2013 for four soil types. The Koc for 615M04 was estimated to be in the range 16 to 28 for a single soil of pH 7. The extent of adsorption was found to decrease as the pH of the soil increased, which is significant for the dicarboxylic acids 615M03 and 615M04. In aged residue leaching studies, 9% of the aged residue was detected in the leachate consisting of unidentified radioactivity. 62.3% of aged residue was detected in the top 6 cm of soil. A 3 year cropped field lysimeter study was carried out in acidic soil (pH 5.7) applying single and successive treatments at 50 g ai/ha in spring; the total annual rainfall was 800 mm. After a single application, annual mean concentrations of radioactivity were 0.29 to 0.34 μg/L, whilst after two applications, the annual mean concentration was 0.53 μg/L. Neither cinidon-ethyl nor its major soil metabolites were identified in the radioactivity.Fate in aquatic systems:
The rate of hydrolytic degradation at 20°C is pH-dependent with DT50 of 5 days at pH 5, 35 hours at pH 7 and 54 minutes at pH 9.
Cinidon-ethyl undergoes photolysis with a half-life of 2.3 days in sterile water; its half-life in the top 1 cm of water is 0.6 to 1.6 days.
In water/sediment study, the DT50 and DT90 in water are 1.5 - 7 hours and 2 - 3 days, respectively, whilst the DT50 and DT90 for the whole system are 5 hours and 2 days, respectively. The ratio of its distribution in water : sediment systems is 73-94 : 27-6. Cinidon-ethyl is not expected to accumulate in the sediment. -
Transport InformationHazard Class:O (Obsolete as pesticide, not classified)
Porduct NewsMore
Bayer develops alternative to glyphosate herbicide
Colombia’s Constitutional Court bans chlorpyrifos
ADAMA Canada moves forward with lambda-cyhalothrin sales for 2023
Mexico: Import of glyphosate drops from 16,500 tons to 8,200 tons
Glyphosate prices drop 30% in Argentina
One year extension of EU glyphosate approval failed to pass in the latest meeting
Palmer amaranth in two southern U.S. states now resistant to S-metolachlor

 0
0 Subscribe
Subscribe