-
Common NameMecoprop-P
-
中文通用名精2甲4氯丙酸
-
IUPAC(R)-2-(4-chloro-o-tolyloxy)propionic acid
-
CAS(2R)-2-(4-chloro-2-methylphenoxy)propanoic acid
-
CAS No.16484-77-8
-
Molecular FormulaC10H11ClO3
-
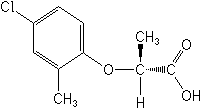
Molecular Structure
-
Category
-
ActivityHerbicide
-
PremixMCPA+dichlorprop-P+mecoprop-P
Aqueous solution (amine), emulsifiable concentrate (ester). Premix Parters: Bacillus cereus; chlormequat chloride ethephon; cyclanilide; ethephon; kinetin; prohexadione-calcium;
-
Physical PropertiesMolecular weight:214.6; Physical form:White crystals, with a weak intrinsic odour. Density:c. 1.31 (20 °C); Melting point:94.6-96.2 °C; ( tech., 84-91 °C); Vapour pressure:0.4 mPa (20 °C); Henry constant:1.0 × 10-4 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 1.43 ( pH 5), 0.02 ( pH 7), -0.18 ( pH 9) (all 20 °C); pKa:3.68 (20 °C); Solubility:In water 860 mg/l ( pH 7, 20 °C). In acetone, diethyl ether, ethanol >1000, dichloromethane 968, toluene 330, hexane 9 (all in g/ kg, 20 °C).; Stability:Stable to heat, light, and stable in the range pH 3 to pH 9. Photolysis DT50 680 h ( pH 5), 1019 h ( pH 7), 415 h ( pH 9).;
-
ToxicologyOral:Acute oral LD50 for rats 1050 mg/kg. Percutaneous:Acute percutaneous LD50 for rats >4000 mg/kg. Eye irritant; not a sensitiser. Inhalation:LC50 (4 h) for rats >5.6 mg/l. Phytotoxicity:Slightly phytotoxic to winter rye (though this is only temporary).
-
Environmental ProfileEcotoxicology:
Algae: EC50 (72 h) for Pseudokirchneriella subcapitata 270, Selenastrum capricornutum 500 mg/l.Bees:Not toxic to honeybees; LD50 (48 h) >25 mg/bee (dimethylammonium salt).Birds:Acute oral LD50 for quail c. 500 mg/kg. Dietary LC50 for bobwhite quail >5600 mg/ kg diet (dimethylammonium salt).Daphnia: EC50 (48 h) >100 mg/l; NOEC (21 d) 50 mg/l.Fish: LC50 (96 h) for trout 150-220, bluegill sunfish >100 mg/l.Worms: LC50 (14 d) for Eisenia foetida 494 mg/ kg soil.Other aquatic spp.: EC50 (14 d) for Lemna minor 60 mg/l.Other beneficial spp.:No effect on Poecilus cupreus or Aleochara bilineata.
Environmental fate:
Animals:In mammals, following oral administration, mecoprop-P is predominantly eliminated as conjugates in the urine.Soil:In soil, degraded predominantly by micro-organisms to 4-chloro-2-methylphenol, followed by ring hydroxylation at the 6-position and ring opening. Soil DT50 (aerobic) 3-13 d.Plant:In plants, mecoprop-P is hydroxylated at the methyl group with formation of 2-hydroxymethyl-4-chlorophenoxypropionic acid. Further metabolism to the carboxylic acid ring may also occur.WATER SOLUBILITY: Tech 1.16 g/l at 20°C -
Transport InformationSignal Word:WARNING; Hazard Class:II(Moderately hazardous)
Porduct NewsMore
EU told not to 'punish' farmers as 'vital' substances set to expire
Benfluralin Dimoxystrobin Fluazinam Flutolanil Mancozeb Mecoprop-P Metiram Oxamyl Pyraclostrobin

 0
0 Subscribe
Subscribe