-
Common NameBentazone
-
中文通用名灭草松
-
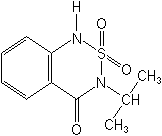
IUPAC3-isopropyl-1H-2,1,3-benzothiadiazin-4(3H)-one 2,2-dioxide
-
CAS3-(1-methylethyl)-1H-2,1,3-benzothiadiazin-4(3H)-one 2,2-dioxide
-
CAS No.25057-89-0
-
Molecular FormulaC10H12N2O3S
-
Molecular Structure
-
Category
-
ActivityHerbicide
-
PremixThifensulfuron-methyl+bentazone+flumetsulam
Penoxsulam+Bentazone
Imazamox+Bentazone
Cyhalofop-butyl+Pyribenzoxim+Bentazone
Bispyribac-sodium+Bentazone
Bentazone+Quinclorac
Bentazone+carfentrazone-ethyl
Acifluorfen+Bentazone
Soluble concentrate (sodium salt), soluble granules. Premix Parters: clomazone; cumyluron thenylchlor;
-
Physical PropertiesMolecular weight:240.3; Physical form:Colourless crystals. Density:1.41 (20 °C); Composition:?/SYM>96% pure. Melting point:139.4-141 °C; Vapour pressure:0.17 (20 °C); Henry constant:7.167 ?/SYM> 10-5 Pa m3 mol-1 (); Partition coefficient(n-octanol and water):logP = 0.77 ( 5), -0.46 ( 7), -0.55 ( 9); pKa:3.3 (24 °C); Solubility:In water 570 /l ( 7, 20 °C). In acetone 1387, methanol 1061, ethyl acetate 582, dichloromethane 206, n-heptane 0.5 ?/SYM> 10-3 (all in g/l, 20 °C).; Stability:Very resistant to hydrolysis in both acidic and alkaline media. Decomposed by sunlight.;
-
ToxicologyOral:Acute oral
50 for rats >1000, dogs >500, rabbits 750, cats 500 / . Percutaneous:Acute percutaneous 50 for rats >2500 /kg. Moderately irritating to skin and eyes (rabbits). Inhalation: 50 (4 h) for rats >5.1 /l air. ADI:( ) 0.1 / Environmental ProfileEcotoxicology:
Algae:50 (72 h) for green algae (Ankistrodesmus) 47.3 /l.Bees:Not toxic to bees; 50 (oral) >100 mg/bee.Birds:Acute oral 50 for bobwhite quail 1140 /kg. Dietary 50 for bobwhite quail and mallard ducks >5000 .Daphnia: 50 (48 h) 125 /l.Fish: 50 (96 h) for rainbow trout and bluegill sunfish >100 /l.Worms: 50 (14 d) >1000 / soil.
Environmental fate:
Animals:Metabolism studies in three different species showed that bentazone was only poorly metabolised, the parent compound being the predominant product. Only small amounts of hydroxylated bentazone metabolites could be detected. No conjugated products were fouSoil:In soil, short-lived hydroxy compounds are first formed, which rapidly undergo further degradation (R. Huber & S. Otto in Rev. Environ. Contam. Toxicol., 137, 111-134). In sunlight, bentazone undergoes rapid degradation, ultimatePlant:In plants, rapidly metabolised to derivatives of anthranilic acid, the principal metabolites being the 6- and 8-hydroxy derivatives, which are conjugated to sugars, forming glycosides.Transport InformationSignal Word:CAUTION;
Hazard Class:III(Slightly hazardous)
Porduct NewsMore
EU renews approval for four pesticide active ingredients
Bentazone Silthiofam Forchlorfenuron Zoxamide
Env MEPs object to herbicide bentazone re-authorisation
Related CompaniesMore
Anhui Fengle Agrochemical Co., Ltd.
Country: China
Tribenuron-methyl Dimethomorph Bentazone Carboxin Quizalofop-P-ethyl Nicosulfuron Clomazone Propiconazole Chlorpyrifos Clopyralid Clodinafop-propargyl
Jiangsu Sword Agrochemicals Co., Ltd.
Country: China
Triadimefon Triadimenol Tebuconazole Diniconazole Bitertanol Cyproconazole Propineb Paclobutrazol Metribuzin Bentazone
Shanghai Nicex International Trading Co.,Ltd.
Country: China
Oxadiazon Oxadiargyl Azoxystrobin TC Bentazone 2,4-D TC MCPA TC Nicosulfuron Tribenuron-methyl Bispyribac-sodium
Ningxia Lamtin Agricultural Development Co.Ltd.
Country: China
Oxadiargyl Bentazone Oxadiazon Metamifop Propanil
Jiangsu Weunite Fine Chemical Co., Ltd.
Country: China
Nitenpyram Azoxystrobin Chlorothalonil Acetamiprid Imidaclorprid Bentazone Carbendazim Chlorfenapyr Chlorthal-dimethyl Metaldehyde

 0
0 Subscribe
Subscribe