-
Common NamePropisochlor
-
中文通用名异丙草胺
-
IUPAC2-chloro-6′-ethyl-N-isopropoxymethylacet-o-toluidide
-
CAS2-chloro-N-(2-ethyl-6-methylphenyl)-N-[(1-methylethoxy)methyl]acetamide
-
CAS No.86763-47-5
-
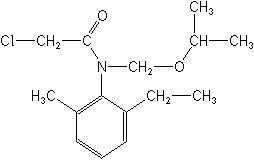
Molecular FormulaC15H22ClNO2
-
Molecular Structure
-
Category
-
ActivityHerbicide.
The product is absorbed by the shoots of germinating plants and inhibits protein synthesis in susceptible plants. Field trials conducted between 1995-98 at the Institute of Plant Protection in Poland on winter wheat, winter barley, sugarbeet, pea, potato and maize showed effective control of Apera spica-venti and Echinochloa crus-galli and improved crop yield. -
CropUseCrop uses:
Apples, beans, cotton, lupins, maize, onions, peanuts, peas, potatoes, rice, soybeans, sugar beet, sunflowers, vines
1,000-2,000 g ai/ha
-
PremixTerbuthylazine+Mesotrione+Propisochlor
Propisochlor+Atrazine
Bensulfuron-methyl+propisochlor
-
Physical PropertiesMolecular weight:283.8; Physical form:Light brown to purple, aromatic oil. Density:1.097 g/cm3 (20 °C); Composition:Tech. is 95% pure. Melting point:21.6 °C; Flash point:175 °C (Marcusson open cup), 110 °C (closed cup); Vapour pressure:4 mPa (20 °C); Henry constant:6.17×10-3 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 3.50 (20 °C); Solubility:In water 184 mg/l (20 °C). Soluble in most organic solvents. Stability:Hydrolytically stable; after 5 d at 50 °C ( pH 4, 7, 9), <10% decomposition. Decomposes above 243 °C. Soluble in most organic solvents. Melting point 21.6°C.
-
ToxicologyOral:Acute oral
LD50 for male rats 3433 mg/kg, female rats 2088 mg/kg. Percutaneous:Acute percutaneous LD50 for male and female rats >2000 mg/kg. Inhalation: LC50 for male and female rats >5000 mg/m3. ADI:( JMPR) 0.007 mg/kg b.w. [1993]. (Rabbit): Mildly irritating to eyes, non-irritating to skin.
-
Environmental ProfileEcotoxicology:
Algae:EC50 for Selenastrum capricornutum 2.8 μg/l.Bees:Non-toxic; LD50 (oral and contact) 100 μg/bee. Birds:Acute oral LD50 for mallard ducks 2000 mg/kg, Japanese quail 688 /kg. LC50 (8 d) for quail and ducks 5000 mg/kg. Daphnia: LC50 (96 h) 6.19 mg/l.Fish: LC50 (96 h) for carp 7.94, rainbow trout 0.25 mg/l.Worms:Not dangerous.Other beneficial spp.:Not dangerous to soil micro-organisms.
Environmental fate:
Animals:In rats, most of applied radioactivity is excreted rapidly, mainly within 24 h.Soil:Decomposed by microbial action; typical soilDT50 10-15 d. Koc 333.3 (acid, sandy soil), 364.4 (acid, loamy soil), 493.5 (alkaline, loamy soil).
WATER SOLUBILITY: 184 mg/l at 20°C. -
Transport InformationSignal Word:CAUTION; Hazard Class:III (Slightly hazardous).
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide
Related CompaniesMore
Shandong Binnong Technology Co., Ltd.
Country: China
Atrazine Ametryn Metolachlor S-metolachlor Terbuthylazine Fomesafen Mesosulfuron-methyl Glufosinate-ammonium Bentazone Pendimethalin
Jiangsu Changlong Agrochemical Co., Ltd.
Country: China
Acetochlor Butachlor Buprofezin Dimethomorph Tebuthiuron Diafenthiuron Methomyl Thiodicarb Imidacloprid Bifenthrin

 0
0 Subscribe
Subscribe