-
Common NamePyrazophos
-
中文通用名吡菌磷
-
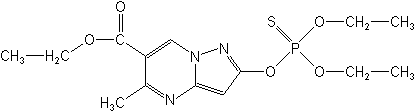
IUPACethyl 2-diethoxyphosphinothioyloxy-5-methylpyrazolo[1,5-a]pyrimidine-6-carboxylate
or
O-6-ethoxycarbonyl-5-methylpyrazolo[1,5-a]pyrimidin-2-yl O,O-diethyl phosphorothioate -
CASethyl 2-[(diethoxyphosphinothioyl)oxy]-5-methylpyrazolo[1,5-a]pyrimidine-6-carboxylate
-
CAS No.13457-18-6
-
Molecular FormulaC14H20N3O5PS
-
Molecular Structure
-
Category
-
ActivityFungicide
-
PremixEmulsifiable concentrate.
Premix Parters: chlorpropham piperonyl butoxide; lindane; permethrin; pheromone; piperonyl butoxide; rotenone;
-
Physical PropertiesMolecular weight:373.4; Physical form:Colourless crystals. Density:1.348 at 25 °C; Composition:Tech. grade is >94% pure. Melting point:51-52 °C; Flash point:34 °C (Abel-Pensky closed method); Vapour pressure:0.22 mPa (50 °C) (Antoine); Henry constant:2.578 × 10-4 Pa m3 mol-1 (calc.); Partition coefficient(n-octanol and water):logP = 3.8; Solubility:In water 4.2 mg/l (25 °C). Readily soluble in most organic solvents, e.g. xylene, benzene, carbon tetrachloride, dichloromethane, trichloroethylene. In acetone, toluene, ethyl acetate >400, hexane 16.6 (all in g/l, 20 °C).; Stability:Hydrolysed by acids and alkalis. Unstable in undiluted form.
-
ToxicologyOral:Acute oral LD50 for rats 151-778 mg/ kg (depending on sex and carrier). Percutaneous:Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin; slightly irritating to eyes (rabbits). Inhalation: LC50 (4 h) for rats 1220 mg/m3 air. Phytotoxicity:Non-phytotoxic when used as recommended (except to some varieties of grape). ADI:(Japan) 0.006 mg/ kg.
-
Environmental ProfileEcotoxicology:
Algae: LC50 (72 h) for Scenedesmus subspicatus 65.5 mg/l.Bees: LD50 (24 h, contact) 0.25µg/bee.Birds:Acute oral LD50 for quail (depending on carrier and sex) 118-480 mg tech./kg. Dietary LC50 (14 d) for mallard ducks c. 340, bobwhite quail c. 300 50 (48 h) 0.36µg/l (soft water), 0.63µg/l (hard water).50 (96 h) for carp 2.8-6.1, rainbow trout 0.48-1.14, bluegill sunfish 0.28 mg/l.Worms: LC50 (14 d) for Eisena foetida >1000 mg/ kg soil.Other beneficial spp.:'Afugan 30 EC' rated harmful to Poecilus cupreus, Syrphus corollae, Chrysoperla carnea and Aleochara bilineata; harmless to Pardosa amentata (IOBC scheme).
Environmental fate:
Animals:Extensively metabolised and rapidly eliminated in rats, DT50 4-5 h. Excretion mainly in urine; the most important metabolite is ethyl 2-hydroxy-5-methyl-6-pyrazolo[1,5-a]pyrimidine-carboxylate, partly excreted as a sulfSoil:Soil degradation occurs by cleavage of the phosphoric acid group, saponification of the carboxylate fraction and further degradation of the heterocyclic ring system, ultimately to CO2. Degradation rates vary with soil type and properties, with Plant:Half-life in wheat leaves c. 19 d. Following hydrolysis of the phosphate bond, ab-glucoside of the pyrazolopyrimidine is formed. WATER SOLUBILITY: 4.2 mg/l at 25°C -
Transport InformationSignal Word:WARNING; Hazard Class:II(Moderately hazardous)
Porduct NewsMore
Bayer develops alternative to glyphosate herbicide
Colombia’s Constitutional Court bans chlorpyrifos
ADAMA Canada moves forward with lambda-cyhalothrin sales for 2023
Mexico: Import of glyphosate drops from 16,500 tons to 8,200 tons
Glyphosate prices drop 30% in Argentina
One year extension of EU glyphosate approval failed to pass in the latest meeting
Palmer amaranth in two southern U.S. states now resistant to S-metolachlor

 0
0 Subscribe
Subscribe