-
Common NameQuinoxyfen
-
中文通用名苯氧喹啉
-
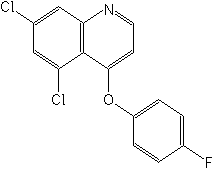
IUPAC5,7-dichloro-4-quinolyl 4-fluorophenyl ether
-
CAS5,7-dichloro-4-(4-fluorophenoxy)quinoline
-
CAS No.124495-18-7
-
Molecular FormulaC15H8Cl2FNO
-
Molecular Structure
-
Category
-
ActivityFungicide
Quinoxyfen inhibits the formation of Erysiphe graminis appressoria. It is a systemic fungicide with additional vapour phase activity, which aids redistribution in all crops. When applied at the first node stage of cereals (GS 31), it is rapidly absorbed by the plant. The activity of quinoxyfen has been attributed to its ability to disrupt a number of signalling processes that are important in the early stages of powdery mildew development (BCPC, 2000, pp 841). The presence of multiple targets reduces the probability that resistance will develop.
The product should not be used on cucurbits grown in glasshouses since some crop damage has been observed under these conditions.No cross-resistance with other mildewicides (azoles, morpholines, pyrimidines) has been noted – this may be due to the ability of quinoxyfen to block a number of pathways in the pathogen's lifecycle. Dow AgroSciences compiled a resistance management strategy, which includes not developing a seed treatment containing the fungicide and not applying after GS 49. The fungicide has been shown not to affect fermentation or the quality of the wine produced from vines treated with it.
The product can provide residual activity lasting for up to 10 weeks. A further application may be made at GS 49 to ensure flag leaf and ear protection. It gives long-term protection of new growth. Quinoxyfen should be applied at 10 - 14 day intervals on vines and at 7 - 10 day intervals on vegetables. It is rainfast within one hour of application. Dow AgroSciences recommends that it is not applied to advanced mildew infestations because it is primarily a protectant treatment. As this product has no curative properties it will not control latent or established infections of powdery mildew present at application. If infection is established, the product can be tank mixed with a curative fungicide from the following compatible products: cyproconazole, epoxiconazole, fenpropidin, fenpropimorph, flusilazole, prochloraz, propiconazole, tebuconazole or tridemorph.
Laboratory and field trials have shown that quinoxyfen has no detrimental effect against beneficial, non-target species and is, therefore, a suitable candidate for use in IPM programs (BCPC, 2000, pp 371).
-
CropUseCROP USES:
barley, cucurbits, hops, melons, oats, peppers, pome fruits, rye, strawberries, triticale, vines, wheat
Cereals
150-300 g ai/ha
Fruit
25-50 g ai/hl; 75-100 g ai/ha
-
PremixTypeAI concnSuspension concentrate (SC)25% (w/w)50% (w/w)
-
Physical PropertiesMolecular weight:308.1g/mol;Off-white solid. Melting point:106-107.5℃;V.p. 1.2×10-2mPa (20℃); 2.0×10-2mPa (25℃).KOWlogP = 4.66 (pH c. 6.6, 20℃);Henry 3.19×10-2Pa m3mol-1(calc.);S.g./density 1.56;Solubility In water 116 g/l (pH 6.45, 20℃). In acetone 116, dichloromethane 589, ethyl acetate 179, methanol 21.5, n-octanol 37.9, toluene 272, hexane 9.64, xylene 200 (all in g/l, 20℃).Stability In dark at 25℃, stable to hydrolysis at pH 7 and 9; DT5075 d (pH 4).
-
ToxicologyOral Acute oral LD50 for rats >5000 mg/kg.Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg.Mild eye irritant, not a skin irritant (rabbits).Skin sensitisation (guinea pigs) depends on the test.Inhalation LC50 for rats >3.38 mg/l.
-
Environmental ProfileECOTOXICOLOGY
Birds LC50 (5 d) >5620 mg/kg diet (2 species).Fish LC50 (96 h) for rainbow trout and bluegill sunfish >0.2 mg/l.Daphnia EC50 (48 h) 0.08 mg/l.Algae EbC50 (72 h) 0.03 mg/l.Bees LC50 (oral) >1000 mg/kg honey.Worms LC50 (14 d) >923 mg/kg soil. Other beneficial spp. Harmless to 4 beneficial insect species (IOBC). Little or no effect in field studies; no effect on soil micro-organisms at 4000 g/ha. See M. Miles & C. Longhurst Proc. Br. Crop Prot. Conf. - Pests Dis., 2000, 1, 371.
ENVIRONMENTAL FATE
Animals Metabolism in goat and hen studied (G. L. Reeves et al., Proc. Br. Crop Prot. Conf. - Pests Dis., 1996, 3, 1169).Plants Only slightly metabolised in wheat, with low residues found in the crop (idem, ibid.). Extensively photodegraded on the wheat leaf surface, giving multiple polar degradation products. On grapes and cucumbers, the main residue was unchanged quinoxyfen (R. Baloch et al., Proc. 9th IUPAC Int. Congr. Pestic. Chem., London, 1998, 5A-008). -
Transport InformationHazard Class:III(Slightly hazardous)
Porduct NewsMore
EU to phase out fungicides quinoxyfen, propiconazole and herbicide flurtamone
Quinoxyfen Propiconazole Flurtamone
Australia approved 13 ais registrations (Special gazette, 9 December 2014)
Quinoxyfen Dimethomorph Trifloxystrobin Isoxaflutole Glufosinate-ammonium Hexazinone Fipronil Clothianidin Thiram Prothioconazole Thiacloprid Chlormequat chloride

 0
0 Subscribe
Subscribe