-
Common NameBromopropylate
-
中文通用名溴螨酯
-
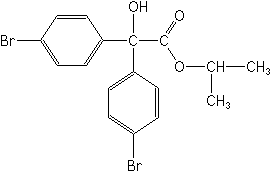
IUPACisopropyl 4,4′-dibromobenzilate
-
CAS1-methylethyl 4-bromo-α-(4-bromophenyl)-α-hydroxybenzeneacetate
-
CAS No.18181-80-1
-
Molecular FormulaC17H16Br2O3
-
Molecular Structure
-
Category
-
ActivityAcaricide/Miticide
-
PremixEmulsifiable concentrate. Premix Parters: atrazine; benazolin ioxynil; bentazone; 2,4-D; 2,4-D propanil; dicamba mecoprop; diflufenican ioxynil; ethofumesate ioxynil; fluroxypyr ioxynil; ioxynil; ioxynil isoproturon; ioxynil isoproturon mecoprop; ioxynil MCPA; ioxynil mecoprop; ioxynil mecoprop-P; MCPA; MCPA mecoprop; terbuthylazine;
-
Physical PropertiesMolecular weight:428.1; Physical form:White crystals. Density:1.59 at 20 °C; Melting point:77 °C; Vapour pressure:6.8 × 10-3 mPa(20 °C); Henry constant:<5.82 × 10-3 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 5.4; Solubility:In water <0.5 mg/l (20 °C). In acetone 850, dichloromethane 970, dioxane 870, benzene 750, methanol 280, xylene 530, isopropanol 90 (all in g/kg, 20 °C).; Stability:Fairly stable in neutral or slightly acidic media; DT50 34 d ( pH 9).;
-
ToxicologyOral:Acute oral LD50 for rats >5000 mg Percutaneous:Acute percutaneous LD50 for rats >4000 mg/kg. Slightly irritating to skin; non-irritating to eyes (rabbits). Inhalation: LC50 for rats >4000 mg/kg. Phytotoxicity:Slightly phytotoxic to certain varieties of apple, plum, and ornamentals.
-
Environmental ProfileEcotoxicology:
Algae: EC50 (72h) for Scenedesmus subspicatus >52 mg/l.Bees:Not toxic to bees; LC50 (24 h) 183mg/bee.Birds:Acute oral LD50 for Japanese quail >2000 mg/kg. Dietary LC50 (8 d) for Pekin ducks 600, Japanese quail 1000 mg/kg diet.Daphnia:LC50 (48 h) 0.17 mg/l.Fish: LC50 (96 h) for rainbow trout 0.35, bluegill sunfish 0.5, carp 2.4 mg/l.Worms: LC50 (14 d) for earthworms >1000 mg/kg soil.Other beneficial spp.:Safe on the relevant adult and immature stages of anthocorids, mirids, coccinellids, Chrysopa, Hemerobius, staphylinids, carabids, syrphids and dolichopodids in deciduous fruits, citrus and hops. The potential hazard to predatory mites c
Environmental fate:
Animals:Bromopropylate is rapidly and efficiently eliminated in animals. Metabolism occurs by cleavage of the isopropyl ester and, to a minor extent, by oxidation. Metabolites formed after oxidation were 3-hydroxybenzilate and conjugates.Soil:The principal metabolite in soil is 4,4-dibromobenzilic acid. For details of persistence in soil, see J. Environ. Qual., 1973, 2, 115. DT50 c. 40-70 d (lab. and field studies). Low mobility in Plant:Studies with 14C-labelled bromopropylate showed little penetration into leaves or fruit. Degradation was slow. -
Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide
Related CompaniesMore
Zhejiang Heben Pesticide & Chemicals Co., Ltd.
Country: China
Difenoconazole Fentin acetate Fentin hydroxide Metalaxyl Propamocarb hydrochloride Propiconazole Penconazole Triflumizole Hexythiazox Metalaxyl-M Clomazone Bromoxynil octanoate Bromopropylate Benalaxyl

 0
0 Subscribe
Subscribe