-
Common NameTri-allate
-
中文通用名野麦畏
-
IUPACS-2,3,3-trichloroallyl diisopropyl(thiocarbamate)
-
CASS-(2,3,3-trichloro-2-propenyl) bis(1-methylethyl)carbamothioate
-
CAS No.2303-17-5
-
Molecular FormulaC10H16Cl3NOS
-
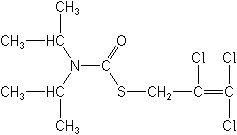
Molecular Structure
-
Category
-
ActivityHerbicide
-
Physical PropertiesMolecular weight:304.7; Physical form:Dark yellow to brown solid; (>30 °C, clear brown to dark brown liquid). Density:1.273 (25 °C); Composition:Tech. is c. 96% pure. Melting point:29-30 °C; Flash point:>150 °C (closed cup); Vapour pressure:16 mPa (25 °C); Henry constant:1.22 Pa m3 mol-1 (calc.); Partition coefficient(n-octanol and water):logP = 4.6; Solubility:In water 4 mg/l (25 °C). Readily soluble in common organic solvents such as acetone, diethyl ether, ethyl acetate, ethanol, benzene, heptane.; Stability:Stable under normal storage conditions. Hydrolysed by strong acids and alkalis. Stable to light. Decomposition temperature >200 °C.;
-
ToxicologyOral:Acute oral LD50 for rats 1100 mg/kg. Percutaneous:Acute percutaneous LD50 for rabbits 8200 mg/kg. Slightly irritating to skin and eyes (rabbits). Not a skin sensitiser. Inhalation:Inhalation of saturated air (5.3 mg/l) for 12 h had no harmful effect on rats. Phytotoxicity:Oats are susceptible. ADI:( JMPR) 0.05 mg/kg b.w. [1989].
-
Environmental ProfileEcotoxicology:
Algae: EC50 (96 h) for Selenastrum capricornutum 0.12 mg/l.Bees:Non-toxic to bees.Birds:Acute oral LD50 for bobwhite quail >2251 mg/kg. Dietary LC50 (8 d) for mallard ducks and bobwhite quail >5620 mg/kg diet.Daphnia: LC50 (48 h) 0.43 mg/l.Fish: LC50 (96 h) for rainbow trout 1.2, bluegill sunfish 1.3 mg/l.Other beneficial spp.:No adverse effect on soil microflora.
Environmental fate:
Animals:Tri-allate metabolism in rats proceeds via three pathways: S-oxidation to sulfur acids, S-oxidation/reduction to thiol derivatives, and C-oxidation of the 2,3,3-trichloropropenethiol moiety.Soil:Main loss from soil is by microbial action (or by volatilisation, if not incorporated in soil). Metabolism proceeds via hydrolytic cleavage with the formation of dialkylamine, CO2 and mercaptan moieties. The latter is transformed via sulfhydrylPlant:2,3,3-Trichloropropenesulfonic acid is the major detectable crop metabolite. -
Transport InformationHazard Class:III(Slightly hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide
Related CompaniesMore
Jiangsu Aolunda High-tech Industry Co.,Ltd
Country: China
Tri-allate EPTC Thiobencarb Molinate Prosulfocarb Dicamba Cyhalofop-butyl Diclosulam Spirodiclofen

 0
0 Subscribe
Subscribe