-
Common NameIndoxacarb
-
中文通用名茚虫威
-
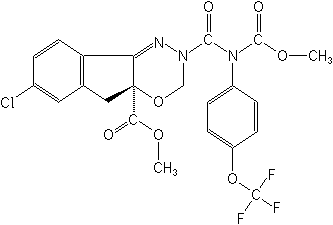
IUPACmethyl 7-chloro-2,3,4a,5-tetrahydro-2-[methoxycarbonyl(4-trifluoromethoxyphenyl) carbamoyl]indeno[1,2-e][1,3,4]oxadiazine-4a-carboxylate
-
CASmethyl 7-chloro-2,5-dihydro-2-[[(methoxycarbonyl)[4-trifluoromethoxy)phenyl]amino] carbonyl]amino]carbonyl]indeno[1,2-e][1,3,4]oxadiazine-4a(3H)-carboxylate
-
CAS No.173584-44-6
-
Molecular FormulaC22H17ClF3N3O7
-
Molecular Structure
-
Category
-
ActivityInsecticide
Indoxacarb is active following contact and ingestion. It acts by blocking the sodium channels in nerve cells (Br. J. Pharmacol., 2001). It has larvicidal and ovicidal activity. Following application, larvae cease feeding within 3-4 hours and subsequently die. The product controls pests resistant to existing insecticides and has a low impact on predatory mites and beneficials, making it suitable for use in IPM schemes and resistance management programmes. Its mode of action is not shared by other insecticides and no cross-resistance with pyrethroids, organophosphates and carbamates has been observed.
It is reported that indoxacarb is not readily broken down by strong UV exposure and is effective under high temperatures. In addition it is rainfast and strongly adherent to the leaf surface. The product is not systemic but shows translaminar movement into the mesophyll. Indoxacarb is recommended for use up to four times per season. DuPont recommends that the product is used in rotation with other pesticides of different mode of action to avoid potential resistance problems in bollworms.
Indoxacarb is particularly effective for the control of bollworm (Helicoverpa armigera) in cotton and does not adversely affect non-target arthropods. In the US, Steward controls the budworm and bollworm complex in the Mid-South cotton states and beet armyworms in Texas, Oklahoma and California. The product is positioned as the only lepidoptera control agent that also controls tarnished plant bugs.
In tests carried out in Canada, populations of fruit tree leafroller (Archips argyrospila) and European leafroller (A rosana) were obtained from 8 sites in the Okanaga Valley, British Columbia and evaluated for their response to a number of insecticides using leaf disc bioassays and neonate larvae (Can. Entom., 2003; J. Econ. Entom., 2002). A range of lethal concentration ratios (LCR; calculated from all populations compared to the most susceptible) was observed for indoxacarb (Avaunt; LCR = 1.59 - 2291.4) whereas spinosad was equally potent against all populations (LCR = 1.92 - 1.98). The high level of resistance to indoxacarb in one population prompted the authors to suggest baseline tolerance screening should be conducted prior to introduction into Canadian orchards.
Indoxacarb was evaluated in autumn 2002 for the broadcast control of red imported fireants (Solenopsis invicta) in Texas. Good control was achieved by the bait within a week.
The Australian Wine Research Institute states that Avatar may be used up to the commencement of bunch closure (berries of 7 mm diameter) and no later than eight weeks before the harvest of the grapes.
In field trials carried out in Texas against the eggs and larvae (first, second, third and fourth instars) of cabbage looper, indoxacarb was found to have little ovicidal effect, but was highly toxic to the larvae. Field-aged leaf residues of indoxacarb were highly toxic to the second (100% mortality 2 days after exposure) and third (100% mortality after 5 d) instars after ingestion. Indoxacarb can be applied three times per season to cabbage, and was found to be as effective as spinosad (P0066) and chlorfenapyr (P0031), and significantly more effective than tebufenozide (P0027L) and emamectin benzoate (P0028). -
CropUseCropUses:
alfalfa, apples, apricot, aubergines, brassicas, broccoli, brussel sprouts, cabbages, cauliflower, cherries, chickpeas, collards, cotton, cranberries, leafy vegetables, fruiting vegetables, grapes, horticultural crops, kohlrabi, lettuce, maize, mungbeans, nashi pears, nectarine, peaches, peanuts, pears, peppers, plums, potatoes, soybeans, sweet corn, tomatoes, top fruits, vines
Cotton
250-335 g ai/ha
Cabbage
0.072 g ai/ha
-
PremixThiamethoxam+Indoxacarb
Tebufenozide+Indoxacarb
Metaflumizone+Indoxacarb
Indoxacarb+Pymetrozine
Indoxacarb+Chlorbenzuron
Indoxacarb+Abamectin
Imidacloprid+indoxacarb
Emamectin benzoate+Indoxacarb
Bifenthrin+Indoxacarb
Bacillus thuringiensis+Indoxacarb
Alpha-cypermethrin+Indoxacarb
-
Physical PropertiesMolecular weight:527.8; Physical form:White powder. Density:1.44 (20°C); Composition:DPX-JW062 is a 1:1 mixture of the (active) (S)- and (inactive) (R)- isomers; DPX-MP062 is a 3:1 mixture of these isomers. The pure (S)- isomer is referred to as DPX-KN128, the (R)- isomer as DPX-KN127. Unless otherwise Melting point:88.1 °C; Vapour pressure:2.5 × 10-5 mPa (25°C); Henry constant:6.0 × 10-5 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 4.65; Solubility:(DPX-KN128) In water 0.20 mg/l (25 °C). (DPX-MP062) In n-octanol 14.5 g/l, methanol 103 g/l, acetonitrile 139 g/l, acetone >250 g/ kg (25 °C).; Stability:Aqueous hydrolysis DT50 >30 d ( pH 5), 38 d ( pH 7), 1 d ( pH 9).
-
ToxicologyOral:DPX-MP062: Acute oral LD50 for male rats 1732, female rats 268 mg/ kg. Percutaneous:DPX-MP062: Acute percutaneous LD50 for rabbits >5000 mg/kg. No eye or skin irritation (rabbits). Dermal sensitiser (guinea pigs). Inhalation:DPX-KN128: LC50 for rats >2 mg/l.
-
Environmental Profile
Ecotoxicology:
Bees:DPX-MP062: LD50 (oral) 23.33 μg/bee; (contact) 1.34 μg/bee.Birds:DPX-MP062: Acute oral LD50 for bobwhite quail 98 mg/kg. Dietary LC50 (5 d) for mallard ducks >5620, bobwhite quail 808 ppm.Daphnia:DPX-MP062: LC50 (48 h) 0.60 mg/l.Fish:DPX-MP062: LC50 (96 h) for bluegill sunfish 0.9, rainbow trout 0.65 mg/l.Worms:DPX-MP062: LC50 (14 d) >1250 mg/ kg.Other beneficial spp.:DPX-KN128 had little or no adverse effects on 4 species at 30-50 g/ ha; more extended studies on Episyrphus balteatus and Typhlodromus pyri described (M. Mead-Briggs et al., Proc. Br. Crop Prot. Conf.
Environmental fate:
Animals:Metabolism in rats after oral dosing was studied using both DPX-JW062 and DPX-MP062. Most of the dose was excreted within 96 h. Extensive metabolism to numerous minor metabolites occurs. In urine, metabolites were cleaved products. Soil: DT50 17 d in tama silt loam soil. DT50 for aquatic photolysis 3.8 d ( pH 5.0).
Fate in aquatic systems:
DPX-JW062 undergoes aqueous hydrolysis and photolysis in water. The half-life decreases with increasing pH. -
Transport InformationHazard Class:O (Obsolete as pesticide, not classified)
Porduct NewsMore
Syngenta introduces Advion WDG insecticide in US
DuPont's Avatar insecticide authorized for application on 38 crops
High chiral ratio indoxacarb technical launched by Jingbo Agrochemical
India CIBRC 350th meeting approved 19 pesticide technical registrations
Tricyclazole Myclobutanil Metconazole Thiamethoxam Buprofezin Indoxacarb Omethoate Spirotetramat Metribuzin Imazethapyr Pyrazosulfuron-ethyl Propyzamide Metamitron Diflubenzuron Quinalphos
Related CompaniesMore
Anhui Fengle Agrochemical Co., Ltd.
Country: China
Tribenuron-methyl Dimethomorph Bentazone Carboxin Quizalofop-P-ethyl Nicosulfuron Clomazone Propiconazole Chlorpyrifos Clopyralid Clodinafop-propargyl
Yancheng Limin Chemical Co.,Ltd
Country: China
Triadimefon Triadimenol Hexaconazole Flutriafol Imidacloprid Paclobutrazol Tebuconazole Thifluzamide Indoxacarb Uniconazole
Shanghai Heben-Eastsun Medicaments Co., Ltd.
Country: China
Thiamethoxam Azoxystrobin Boscalid Chlorfenapyr Difenoconazole Fludioxonil Indoxacarb Picoxystrobin Pyraclostrobin Tebuconazole
Shandong Jingbo Agrochemicals Technology Co., Ltd.
Country: China
Pyrimethanil Pyraclostrobin Fomesafen Tebufenozide Fenoxanil Azoxystrobin Kresoxim-methyl Boscalid Nicosulfuron Emamectin benzoate Indoxacarb Quizalofop-P-ethyl
Zhejiang Xinnong Chemical Co., Ltd.
Country: China
Pyraclostrobin +Zinc Thiazole Pyraclostrobin+Boscalid Pyraclostrobin +Epoxiconazole Pyraclostrobin Chlorpyrifos+cypermethrin Chlorpyrifos Tebuconazole +Zinc thiazole Azoxystrobin+Zinc thiazole Kasugamycin +Zinc thiazole Zinc thiazole

 0
0 Subscribe
Subscribe