-
Common NameEthion
-
中文通用名乙硫磷
-
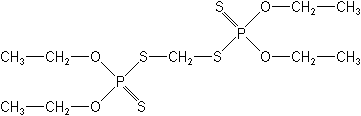
IUPACO,O,O',O'-tetraethyl S,S'-methylene bis(phosphorodithioate)
-
CASS,S'-methylene bis(O,O-diethyl) phosphorodithioate
-
CAS No.563-12-2
-
Molecular FormulaC9H22O4P2S4
-
Molecular Structure
-
Category
-
ActivityAcaricide/Miticide
-
PremixCypermethrin+ethion
Dust, emulsifiable concentrates, emulsifiable solution, granules, wettable powder.
Premix Parters: bromoxynil ioxynil; chloridazon; chlorpyrifos alpha-cypermethrin metamitron; desmedipham phenmedipham; lenacil; lenacil phenmedipham; metamitron; metamitron phenmedipham; phenmedipham;
-
Physical PropertiesMolecular weight:384.5; Physical form:Water-white to amber-coloured liquid. Density:1.22 (20 °C); ( tech., 1.215-1.230); Melting point:-15 to -12 °C; Flash point:176 °C (Pensky-Martens closed cup); Vapour pressure:0.20 mPa (25 °C); Henry constant:3.85 × 10-2 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 4.28; Solubility:In water 2 ppm (25 °C). Miscible with most organic solvents, e.g. acetone, methanol, ethanol, xylene, kerosene, petroleum oils.; Stability:Hydrolysed by aqueous acids and alkalis; DT50 390 d ( pH 9). Slowly oxidised by air.
-
ToxicologyOral:Acute oral LD50 for rats 208 mg/ kg (pure), 21 mg/ kg ( tech.); for mice and guinea pigs 40-45 mg/ kg. Percutaneous:Acute percutaneous LD50 for rats 838 mg/ kg, guinea pigs and rabbits 915 mg/ kg; ( tech., for rabbits 1084 mg/ kg Inhalation: LC50 (4 h) for rats 0.45 mg tech./l. Phytotoxicity:Non-phytotoxic when used as directed, except to some varieties of apple (particularly early-maturing varieties, like Early McIntosh). ADI:( JMPR) 0.1 mg/ kg b.w. [1982].
-
Environmental ProfileEcotoxicology:
Bees:Toxic to bees.Birds: LD50 for quail 128, ducks >2000 mg tech./ kg.Daphnia: EC50 (48 h) 0.056 μg/l.Fish:Toxic to fish. Average lethal concentration 0.72 mg/l (24 h); 0.52 mg/l (48 h).
Environmental fate:
Animals:Metabolised in animals by oxidation to a phosphorothioate, followed by dealkylation and hydrolysis.Soil:Typical DT50 in soil 90 d.Plant:Break down is slow, and caused by weathering, rather than by plant metabolism. -
Transport InformationSignal Word:WARNING; Hazard Class:II(Moderately hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide
Related CompaniesMore
Country: India
Cypermethrin Permethrin Alpha-cypermethrin Fenvalerate Lambda-cyhalothrin Chlorpyrifos-methyl Ethion Phenthoate Bifenthrin Thiamethoxam
Country: India

 0
0 Subscribe
Subscribe