-
Common NameMethoxyfenozide
-
中文通用名甲氧虫酰肼
-
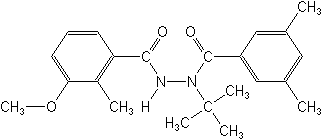
IUPACN-tert-butyl-N'-(3-methoxy-o-toluoyl)-3,5-xylohydrazide
-
CAS3-methoxy-2-methylbenzoic acid 2-(3,5-dimethylbenzoyl)-2-(1,1-dimethylethyl)hydrazide
-
CAS No.[161050-58-4]
-
Molecular FormulaC22H28N2O3
-
Molecular Structure
-
Category
-
ActivityInsecticide.Methoxyfenozide is an insect growth regulator, which is absorbed in the haemolymph of insect larvae following ingestion and binds to the ecdysone receptor specific for 20-hydroxyecdysone, which normally regulates the moulting process. Binding by methoxyfenozide rapidly induces a premature moulting process in target pests; the affinity for the lepidopteran ecdysone receptor complex is significantly higher than other diacylhydrazines (Kd for Plodiareceptor = 0.5 nM compared with 3 nM for tebufenozide and 150 nM for halofenozide). The larvae cannot shed the old cuticle, feeding is prevented and the larvae die by starvation. The action of methoxyfenozide is highly specific to lepidopteran species; the lack of activity on beneficials such as honeybees, parasitoids and predators (J. Econ. Entomology, 2000) makes methoxyfenozide a potential candidate for use in IPM programmes.
Activity is largely expressed through ingestion of treated plant tissue though some contact activity has been noted. Some translaminar movement occurs. Ovicidal efficacy has been noted for certain species such as Lobesia botrana, Cydia pomonella, Choristoneura rosaceana.
Timing of application is important; for larvae which feed inside the plant tissue, the application should be just prior to egg-hatching in order to kill the insects while they feed at the surface whereas treatment of permanent surface-feeders is made during the feeding phase. Optimum activity is observed when the applications are made to early instars.
Methoxyfenozide provides up to 2 weeks residual activity against targets such as beet armyworms but the lack of leaf systemicity means that reapplication is necessary to cover new leaf growth. The reapplication interval depends on the rate of crop growth and the duration of the infestation. A maximum of three treatments per annum is recommended on grapes for wine and four on grapes to be consumed as fruit; the last application should be applied 14 days before the harvest of grapes for wine and 7 days for fruit. For cotton, the crop should not be harvested for 4 weeks after application.
Optimal coverage and adhesion to the leaf surface is obtained by the use of spray adjuvants. The company recommend Latron B-1956, Latron CS-7 or crop-based oils.
There is no evidence to date that suggests any cross-resistance between methoxyfenozide and other classes of insecticides with different modes of action.
In field trials conducted in British Columbia (J Econ. Entomol., 2002), resistance to methoxyfenozide of neonate F1 progeny of Choristoneurarosaceanafrom organic and conventionally managed orchardswas determined using a leaf disk bioassay. Insects collected from organic sites were found to be more susceptible than insects collected from conventional sites. Three other insecticides were tested: azinphosmethyl, tebufenozide (P0027L) and indoxacarb (P0076); cross-resistance between methoxyfenozide and azinphosmethyl was found across populations.
Residual activity and toxicity of methoxyfenozide to codling moth, (Cydia pomonella), and oriental fruit moth (Grapholita molesta) were investigated in North Carolina apples (J. Econ. Entomol., 2004). Methoxyfenozide exhibited greater activity than tebufenozide against codling moth eggs with approx. 5-fold lower LC50 values to eggs laid on fruit treated before or after oviposition. Oriental fruit moth eggs were 57-fold less sensitive to methoxyfenozide than codling moth eggs on fruit treated before oviposition, and 12-fold less sensitive when treated afterwards. Methoxyfenozide reduced larval entries of both codling moth and oriental fruit moth for at least 28 days after application. Residue breakdown on fruit was approx. 80% at 28 days after treatment, with the most rapid residue decline (60%) occurring during the first 14 days after application. Two applications of methoxyfenozide applied at 14-day intervals provided better canopy coverage and higher residue levels than one application. Spray volume did not affect efficacy levels. -
CropUseCropUses:
apples, apricots, artichokes, barley, beans, blueberries, brassicas, bulb vegetables, carrots, cereals, cilantro, citrus, cole crops, coriander, cotton, cranberries, cucurbits, fodder, forage, forestry, fruiting vegetables, leafy vegetables, grasses, herbs, legume vegetables, longans, lychees, maize, mangos, mints, nectarines, nuts, oats, okra, ornamentals, papaya, peaches, pears, peanuts, peas, pistachios, pulasan, radish, rambutans, raspberries, rice, root vegetables, tuber vegetables, rye, soybeans, Spanish limes, spices, strawberries, stone fruits, sugar beets, sweet corn, sweet potatoes, tea, tomatoes, tree nuts, tropical fruit, wheat, vines
56-111 g ai/ha (Spodoptera spp in cotton)
222-333 g ai/ha (Archips spp, Cydia pomonella in pome fruit)
278-389 g ai/ha (Helicoverpa spp)
Apple, pear
0.6 L product/ha
-
PremixSpinetoram+methoxyfenozide
Pymetrozine+Methoxyfenozide
Methoxyfenozide+Pyridalyl
Metaflumizone+Methoxyfenozide
Emamectin benzoate+Methoxyfenozide
Chlorfenapyr+Methoxyfenozide
Abamectin+MethoxyfenozideType
AI concn
Suspension Concentrate (SC)
24% (w/v)
Wettable Powder (WP)
80% (w/w)
-
Physical PropertiesMolecular weight:368.5; Physical form:White powder. Melting point:204-205 °C; Vapour pressure:<1.48 × 10-3 (20 °C); Henry constant:<1.64 × 10-4 Pa m3 mol-1 (); Partition coefficient(n-octanol and water):logP = 3.7 (shake flask); Solubility:In water 3.3 /l. In 11, cyclohexanone 9.9, acetone 9 (all in g/100 g).; Stability:Stable at 25 °C and to hydrolysis at 5, 7 and 9.;
-
ToxicologyOral:Acute oral
LD50 for rats and mice >5000 / . Percutaneous:Acute percutaneous <span lang="EN-US" style="FONT-SIZE: 9pt; FONT-FAMILY: Arial; mso-font-kerning: 1.0pt; mso-ansi-l -
Environmental ProfileEcotoxicology:
Algae:LD50 (96 and 120 h) for Selenastrum >3.4 /l.Bees:Non-toxic (oral and contact) at 100 mg/bee.Birds:Acute oral LD50 for bobwhite quail >2250 /kg. Dietary LC50 (8 d) for mallard ducks and bobwhite quail >5620 / diet.Daphnia: LC50 (48 h) 3.7 /l.Fish: LC50 (96 h) for bluegill sunfish >4.3, rainbow trout >4.2 /l.Worms: LC50 for earthworms (14 d) >1213 / soil.Other beneficial spp.:Not toxic to a range of insect species.
Environmental fate:
Animals:Rapidly absorbed, metabolised via phase II conjugation and eliminated.Soil:Photolytic50 in pond water 77 d. Soil photolysis 50 173 d; aerobic soil metabolism 50 336-1100 d (4 soil types); field 50 23-268 d (10 stu -
Transport InformationHazard Class:O (Obsolete as pesticide, not classified)
Porduct NewsMore
EU proposes to restrict use of methoxyfenozide
Corteva Agriscience launches Intrepid Edge insecticide in Brazil
Related CompaniesMore
SINO AGRO-CHEMICAL INDUSTRY LTD.
Country: China
Prothioconazole Azoxystrobin Pyraclostrobin Methoxyfenozide Bifenazate Bispyribac-sodium Nicosulfuron Tebuconazole Pyribenzoxim Penoxsulam
Jiangsu Huifeng Bio Agriculture Co., Ltd.
Country: China
Prochloraz Prochloraz-manganese Trinexapac-ethyl Bromoxynil octanoate Epoxiconazole Glufosinate-ammonium Diflufenican Methoxyfenozide Bifenthrin Prochloraz-copper Lambda-cyhalothrin
Jiangsu Yongan Chemical Co., Ltd.
Country: China
Pendimethalin Methoxyfenozide 2,4-D Clethodim Quinclorac Glufosinate-ammonium Metolachlor Atrazine Pendimethalin
Jiangyin Milagro Chemical Co.,Ltd
Country: China
Nuclear polyhedrosis virus Agriculural Organosilicone Adjuvant Amino acids seaweed formulation Ethephon Thiamethoxam+lambda-cyhalothrin Azoxystrobin + Difenoconazole Flumioxazin Indoxacarb Potassium Humate Enzymolysis Amino Acid Series
Country: China
MCPA Cyproconazole Imazamox Flumetsulam Haloxyfop-P-methyl Penoxsulam Florasulam Prothioconazole Clothianidin

 0
0 Subscribe
Subscribe