-
Common NameCycloate
-
中文通用名环草敌
-
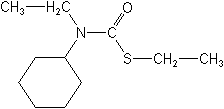
IUPACS-ethyl cyclohexyl(ethyl)thiocarbamate
-
CASS-ethyl cyclohexylethylcarbamothioate
-
CAS No.1134-23-2
-
Molecular FormulaC11H21NOS
-
Molecular Structure
-
Physical PropertiesMolecular weight:215.4; Physical form:Colourless liquid with an aromatic odour. Specific gravity 1.023-1.026 at 20°C. Density:1.024 g/ml; Composition:Tech. material is 95% pure. Flash point:>100 °C; Vapour pressure:2.13 mPa (25 °C); Henry constant:6.12×10-3 Pa m3 mol-1 ( calc., 20 °C); Partition coefficient(n-octanol and water):logP = 3.88; Solubility:In water 75 mg/l (20 °C), Miscible with most organic solvents, e.g. acetone, benzene, methanol, ethanol, xylene, kerosene, etc.; Stability:Thermally stable (120 °C). Hydrolyzed by strong acids and alkalis. Stable to heating to 100°C for 16 hours. Miscible with acetone, benzene, ethanol, kerosene, 4-methylpentan-2-one, xylene.
-
ToxicologyOral:Acute oral
LD50 for male rats 2000-3190 mg/kg, female rats 3160-4100 mg/kg. Percutaneous:Acute percutaneous LD50 for rabbits >5000 mg/kg. Non-irritating to skin and eyes (rabbits). Inhalation: LC50 (4 h) for rats 4.7 mg/l. -
Environmental ProfileEcotoxicology:
Bees:Not toxic to bees at 0.011mg/bee.Birds: LD50 for Japanese quail >2000 mg/kg. Dietary LC50 (7 d) for bobwhite quail >56 000 mg/ kg diet (720 g/l for EC formulation).Daphnia: LC50 (48 h) 5.6 mg/l.Fish: LC50 (96 h) for rainbow trout 4.5 mg/l.
Environmental fate:
Animals:Cycloate is converted to the sulfoxide by microsomal enzymes, and then cleaved rapidly by the liver soluble-glutathione system.Soil:Microbial degradation is responsible for a large part of the disappearance of cycloate from soil.DT50 in soil c. 4-8 w.Plant:Rapidly metabolised in the roots and leaves of beets. Metabolites include ethyl cyclohexylamine, CO2, amino acids, sugars, and other natural plant constituents.
WATER SOLUBILITY: 75 mg/l -
Transport InformationSignal Word:CAUTION; Hazard Class:III (Slightly hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide

 0
0 Subscribe
Subscribe