-
Common NameIprovalicarb
-
中文通用名缬霉威
-
IUPACisopropyl 2-methyl-1-{[(RS)-1-p-tolylethyl]carbamoyl}-(S)-propylcarbamate
-
CAS1-methylethyl [(1S)-2-methyl-1-[[[1-(4-methylphenyl)ethyl]amino]carbonyl]propyl]carbamate
-
CAS No.140923-17-7
-
Molecular FormulaC18H28N2O3
-
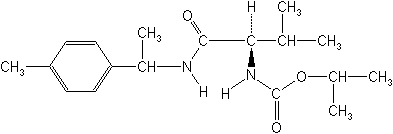
Molecular Structure
-
Category
-
ActivityFungicide
Iprovalicarb is a systemic fungicide specific to oomycetes. After spraying, most of the active substance remains on the plant surface, where it forms a deposit that protects the plant from new infections. A smaller proportion of the active substance is taken up into the plant, after which it is rapidly redistributed to the apical tissues. After drench application, iprovalicarb is rapidly taken up either by the roots or through the plant cuticle and is distributed around the plant in the transpiration stream. The product affects the growth of germ tubes of zoospores, sporangia and mycelia. It also affects sporulation. Adjuvants (such as oils) enhance the uptake by the plant; this is particularly useful under high disease pressure. The rate of uptake is also higher under conditions of high temperature and high humidity (including after rainfall and after re-wetting). Iprovalicarb has protectant, curative and anti-sporulant activity. Given its retention on the plant surface, Bayer is recommending its use only in protectant schedules, mixed with contact fungicides, to extend its disease spectrum and to delay/prevent the onset of resistance. In situations where protective application has not been possible and the first spray of Melody is intended to be curative, it is recommended that subsequent spray intervals be reduced in order to guarantee effective control of the disease.
Field trials were carried out to investigate the post-infection efficiency of curative fungicides against downy mildew on leaves and inflorescences, applying the fungicide either 2 h before artificial infections or at 15%, 30%, 50% and 80% of the incubation process. Whilst all compounds tested worked well on leaves if applied before infection, iprovalicarb showed the best results in all post-infection treatments, although the infected tissue showed necroses. However there was no sufficient curative effect on inflorescences.
Bayer recommends its use in both resistance management and integrated pest management schemes. -
CropUseCropUses:
citrus, cucumbers, gourds, lettuces, melons, onions, peppers, potatoes, tobacco, tomatoes, vegetables, vines, watermelons
120-240 g ai/ha (in mixture with other a.i.'s)
-
PremixIprovalicarb+propineb
Water-dispersible granule (WG) ;Soluble concentrate, wettable granule, wettable powder.
Premix Parters: carbendazim; iprobenfos; propiconazole;
-
Physical PropertiesMolecular weight:320.4; Physical form:White to yellow powder. Density:1.11 (20 °C); Composition:A mixture of (S,S)- and (S,R)- diastereoisomers. Melting point:183 °C (SR); 199 °C (SS); 163-165 °C (mixture); Vapour pressure:4.4 × 10-5 (SR); 3.5 × 10-5 (SS); 7.7 × 10-5 (mixture) (all mPa, 20 °C); Henry constant:1.3 × 10-6 (SR); 1.6 × 10-6 (SS) (both Pa m3 mol-1, 20 °C, calc.); Partition coefficient(n-octanol and water):logP = 3.2 ((SR)- and (SS)- diastereoisomers); Solubility:In water 11.0 ((SR)- diastereoisomer), 6.8 ((SS)- diastereoisomer) (both mg/l, 20 °C).
-
ToxicologyOral:Acute oral LD50 for rats >5000 mg/ kg. Percutaneous:Acute percutaneous LD50 for rats >5000 mg/kg. Non-irritating to skin and eyes (rabbits); not a skin sensitiser (guinea pigs). Inhalation: LC50 for rats >4977 mg/m3 air (dust). ADI:( JMPR) 0.06 mg/ kg b.w. [1995].
-
Environmental Profile
Ecotoxicology:
Algae: ErC50 (72 h) for green algae (Selenastrum capricornutum) >10.0 mg/l.Bees: LD50 (48 h) for honeybees (oral) >199 μg/bee; (contact) >200 μg/bee.Birds:Acute oral LD50 for bobwhite quail >2000 mg/kg. Dietary LC50 (5 d) for bobwhite quail and mallard ducks >5000 mg/ kg feed.Daphnia: EC50 (48 h) >19.8 mg/l.Fish: LC50 (96 h) for rainbow trout >22.7, bluegill sunfish >20.7 mg/l.Worms: LC50 (14 d) for Eisenia foetida >1000 mg/ kg dry soil.Other beneficial spp.:No negative effects on Typhlodromus pyri at 460 g/ ha, on Poecilus cupreus at 2 × 461 g/ha, on Coccinella septempunctata at 550 g/ ha, or on Aphidius rhopalosiphi
Environmental fate:
Animals:After oral administration of radiolabelled iprovalicarb to rats and to lactating goats, the radioactivity was readily excreted via faeces and urine. Iprovalicarb was metabolised extensively;
Fate in soil:
Iprovalicarb has moderate to low mobility in soil. It has a soil half-life of between 1 and 17 days. The major metabolite in soil degradation studies is p-methyl-phenethylamine (PMPA).
In laboratory studies, the DT50 at 20°C in aerobic soil was found to be 2.0 to 29.6 days (median 10.5 days), whilst the DT90 was in the range 6.7 to 98.3 days (median 34.9 days) at the same temperature. Studies in anaerobic soil were not required as the proposed use of iprovalicarb is as a fungicide for fruits and vegetables.
In field studies, the mean DT50 from 6 trials (two in Southern Europe and four in Northern Europe) was found to be 15.5 days; the DT90 ranged from 15.5 to 64.2 days in Southern Europe and averaged 54.7 days in Northern Europe. Iprovalicarb is unlikely to accumulate in soil as it is not persistent.
The metabolite PMPA was found to have a DT50 of 41 to 118 days (median = 52 days) at 20°C in aerobic soils, and a DT90 of greater than 100 days. In field studies, DT50 was found to be 2.2 to 22 days (mean of 12 days).
The mean Koc for iprovalicarb was 106 mL/g and that of the metabolite PMPA was 290 mL/g indicating that they have medium mobility in soil.
Leaching studies and lysimeter studies were not carried out as groundwater contamination by iprovalicarb was not expected.Fate in aquatic systems:
Iprovalicarb is stable to hydrolysis at 25°C in the pH range 5 to 9. It is not susceptible to photodegradation.
In two water/sediment studies, the DT50 and DT90 in water were 54.4 and 18.8 days, and 180.8 and 62.5 days, respectively. For the whole system, the DT50 was 55.7 and 25.3 days, and DT90 185.1 and 83.9 days. -
Transport InformationHazard Class:O (Obsolete as pesticide, not classified)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide

 0
0 Subscribe
Subscribe