-
Common NameBifenox
-
中文通用名甲羧除草醚
-
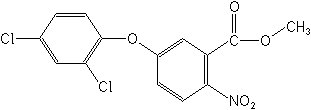
IUPACmethyl 5-(2,4-dichlorophenoxy)-2-nitrobenzoate
-
CASmethyl 5-(2,4-dichlorophenoxy)-2-nitrobenzoate
-
CAS No.42576-02-3
-
Molecular FormulaC14H9Cl2NO5
-
Molecular Structure
-
Category
-
ActivityHerbicide
-
PremixPremix Parters: amitraz; S-bioallethrin; zeta-cypermethrin; imidacloprid; indole-3-butyric acid;
-
Physical PropertiesMolecular weight:342.1; Physical form:Yellow crystals with a slightly aromatic odour. Density:0.65 g/ml (bulk density); Composition:×97% pure. Melting point:84-86 °C; Vapour pressure:0.32 mPa(30 °C); Henry constant:1.14 × 10-2 Pa m3 mol-1; Partition coefficient(n-octanol and water):logP = 4.5; Solubility:In water 0.35 mg/l (25 °C). In acetone 400, chlorobenzene 400, xylene 300, ethanol <50 (all in g/kg, 25 °C). Slightly soluble in aliphatic hydrocarbons.; Stability:Thermally stable up to 175 °C; total decomposition occurs above 290 °C. Stable in slightly acidic or slightly alkaline media, but rapidly hydrolysed above pH 9. DT50 in saturated aqueous solution, 24 m;
-
ToxicologyOral:Acute oral LD50 for rats >5000 mg tech./kg, mice 4556 mg/kg. Percutaneous:Acute percutaneous LD50 for rabbits >2000 mg/kg. Non-irritating to skin and eyes. Inhalation:LC50 for rats >0.91 mg/l air.
-
Environmental ProfileEcotoxicology:
Bees: LD50(contact) >1000mg/bee.Birds:Dietary LC50(8 d) for ducks and pheasants >5000 mg/kg diet.Daphnia:LC50 (48 h) 0.66 mg/l.Fish: LC50(96 h) for rainbow trout >0.67, bluegill sunfish >0.27 mg/l.Worms: EC50 and NOEC >1000 mg/kg.Other aquatic spp.:LC50 (96 h) for Palaemonetes pugio (grass shrimp) 569 ppm, for Mysidopsis bahia (mysid shrimp) 0.065 mg/l; (48 h) for Crassostrea virginica (Eastern oyster) 210
Environmental fate:
Animals:Bifenox is relatively rapidly absorbed and eliminated from the body; 5-(2,4-dichlorophenyl)-2-nitrobenzoic acid was the major urinary metabolite, with no bifenox detected. Bifenox together with 5-(2,4-dichlorophenyl)anthranilate were detected in faeces.Soil:DT50 in soil c. 5-7 d. Duration of residual activity is c. 7-8 w. Degradation is mainly chemical and microbial, and the two principal metabolites are 5-(2,4-dichlorophenoxy)-2-nitrobenzoic acid and methyl 5-(2, -
Transport InformationHazard Class:III(Slightly hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide

 0
0 Subscribe
Subscribe