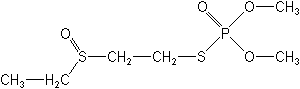-
Common NameOxydemeton-methyl
-
中文通用名亚砜磷
-
IUPACS-2-ethylsulfinylethyl O,O-dimethyl phosphorothioate
-
CASS-[2-(ethylsulfinyl)ethyl] O,O-dimethyl phosphorothioate
-
CAS No.301-12-2
-
Molecular FormulaC6H15O4PS2
-
Molecular Structure
-
Category
-
ActivityInsecticide
-
PremixSpray concentrate.
Premix Parters: copper sulfate, tribasic streptomycin; streptomycin.
-
Physical PropertiesMolecular weight:246.3; Physical form:Colourless liquid. Density:1.289 (20℃); Melting point:<-20℃; Flash point:113℃; Vapour pressure:3.8 mPa(20℃); Henry constant:<<1 × 10-5 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = -0.74 (21℃); Solubility:Completely miscible with water. Soluble in common organic solvents, except in petroleum ether.; Stability:Relatively slowly hydrolysed in acidic media, but rapidly hydrolysed in alkaline media; DT50( est.) 107 d ( pH4), 46 d ( pH7), 2 d ( pH9) (22℃).
-
ToxicologyOral:Acute oral LD50 for rats c. 50 mg/ kg. Percutaneous:Acute percutaneous LD50 for rats c. 130 mg/kg. Mild skin irritant; eye irritant (rabbits; 50% in MIBK). Inhalation: LC50 (4 h) for rats 471 mg/m3 (50% in MIBK). Phytotoxicity:Some ornamentals may be injured, more especially in combination with other pesticides. ADI:0.15 mg/ kg.
-
Environmental ProfileEcotoxicology:
Algae: ErC50 for Scenedesmus subspicatus 49 mg/l.Bees:Toxic to bees.Birds: LD50 for bobwhite quail 34-37 mg/kg. LC50 (5 d) for mallard ducks >5000, bobwhite quail 434 mg/ kg diet.Daphnia: LC50 (48 h) 0.19 mg/l.Fish: LC50 (96 h) for rainbow trout 17, golden orfe 447.3, bluegill sunfish 1.9 mg/l.Worms: LC50 for Eisenia foetida 115 mg/ kg dry soil.
Environmental fate:
Animals:Elimination is very quick; almost 99% is excreted within 48 h in the urine.Soil:Degradation in different soils is extremely rapid. The main metabolic routes involve oxidation of the sulfoxide to a sulfone group, and oxidative and hydrolytic cleavage of the side-chain, with the formation of dimethylphosphoric acid and phosphoric acid.Plant:Oxydemeton-methyl is very quickly metabolised in sugar beet. Besides oxidation to biologically-active demeton-S-methylsulphon, the main metabolic reactions are hydrolysis and subsequent dimerisation, as well as the formation of conjugates. -
Transport InformationHazard Class:Ib(Highly hazardous)
Porduct NewsMore
Bayer develops alternative to glyphosate herbicide
Colombia’s Constitutional Court bans chlorpyrifos
ADAMA Canada moves forward with lambda-cyhalothrin sales for 2023
Mexico: Import of glyphosate drops from 16,500 tons to 8,200 tons
Glyphosate prices drop 30% in Argentina
One year extension of EU glyphosate approval failed to pass in the latest meeting
Palmer amaranth in two southern U.S. states now resistant to S-metolachlor

 0
0 Subscribe
Subscribe