-
Common NameBrodifacoum
-
中文通用名溴鼠灵
-
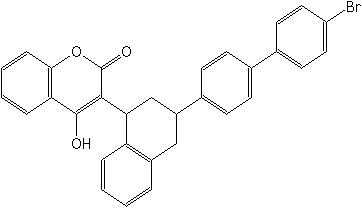
IUPAC3-[(1RS,3RS;1RS,3SR)-3-(4′-bromobiphenyl-4-yl)-1,2,3,4-tetrahydro-1-naphthyl]-4-hydroxycoumarin
-
CAS3-[3-(4′-bromo[1,1′-biphenyl]-4-yl)-1,2,3,4-tetrahydro-1-naphthalenyl]-4-hydroxy-2H-1-benzopyran-2-one
-
CAS No.56073-10-0
-
Molecular FormulaC31H23BrO3
-
Molecular Structure
-
Category
-
ActivityRodenticide.
Brodifacoum, like other anticoagulant toxicants, works byincreasing (or decreasing) the clotting time of blood, leading to death from haemorrhaging. Brodifacoum isabsorbed through the gastrointestinal tract and can alsobe absorbed through the skin -
Pest Spectrumit is used to control hamsters, mice, rats and other rodents which have proved difficult to control with
other anticoagulants. -
FormulationBB = Block bait
RB = Bait
RG =
TKL =
TC = TC
-
Physical PropertiesMolecular weight:523.4; Physical form:White powder; (tech., off-white to buff or beige powder). Density:1.42 (25 °C, tech.); Composition:Tech. material is >91% pure. Melting point:228-232 °C ( tech.); Vapour pressure:<<0.001 mPa (20 °C, gas saturation method); Henry constant:<10-1 ( pH 5.2); <10-3 ( pH 7.4); <10-5 ( pH 9.3) (all in Pa m3 mol-1, calc.); Partition coefficient(n-octanol and water):logP = 8.5; pKa:A very weak acid which is too lipophilic to form water-soluble salts.; Solubility:In water 3.8 × 10-3 ( pH 5.2), 0.24 ( pH 7.4), 10 (pH 9.3) (all in mg/l, 20 °C). In acetone 20, chloroform 3, benzene <6 (all in mg/l, 20 °C).; Stability:Thermally (up to 50 °C) and photolytically (30 d in direct sunlight) stable. Degraded by u.v. light when in solution.;
-
ToxicologyOral:Acute oral LD50 for male rats 0.4, male rabbits 0.2, male mice 0.4, female guinea pigs 2.8, cats c. 25, dogs 0.25-3.6 mg/kg. Percutaneous:Acute percutaneous LD50 for rabbits 0.25-0.63 mg/kg. Slight to mild skin and eye irritant (rabbits). A moderate skin sensitiser (guinea pigs), but negligible risk of sensitisation from formulated product. Inhalation: LC50 (4 h) 5
mg/l air. -
Environmental ProfileEcotoxicology:
Birds:Acute oral LD50 for Japanese quail 11.6, chickens 4.5, mallard ducks 0.31 mg/kg. Dietary LC50 (40 d) for mallard ducks 2.7, bobwhite quail 0.8 ppm.Daphnia: LC50 (48 h) 0.34 mg a.i./l (as formulation).Fish: LC50 (96 h) for bluegill sunfish 0.165, rainbow trout 0.051 mg/l.
Environmental fate:
Animals:In mammals, a number of hydroxycoumarins are formed.Soil:Degraded in soils ( pH 5.5 to pH 8) under aerobic and flooded conditions. Koc (average) 50 000, range 14 000-106 000; Kd 1040 (average), range 625-1320. DT -
Transport InformationSignal Word:DANGER; Hazard Class:Ib(Highly hazardous)
Porduct NewsMore
ECHA to approve eight rodenticides as candidates for substitution
Brodifacoum Bromadiolone Chlorophacinone Coumatetralyl Difenacoum Difethialone Flocoumafen Warfarin
EU proposes to postpone expiry date of approval for three rodenticides
Flocoumafen Brodifacoum Warfarin
Related CompaniesMore
Country: China
Brodifacoum Bromadiolone Difenacoum Chloralose Transfluthrin Tetramethrin Meperfluthrin Bioallethrin Dimefluthrin Prallethrin
Shanghai Agrotree Chemical Co Ltd
Country: China
Acetamiprid Carbofuran Cartap hydrochloride Imidacloprid Monosultap Pyriproxyfen Atrazine Carbendazim Tricyclazole Gibberellic acid
Country: China
ABAMECTIN CARBOFURAN Chlorpyrifos LAMBDA-CYHALOTHRIN 2,4-D GLYPHOSATE ALUMINIUM PHOSPHIDE EDDHA-Fe 6% ZINC SULFATE HEPTAHYDRATE

 0
0 Subscribe
Subscribe