-
Common NamePiperonyl butoxide
-
中文通用名增效醚
-
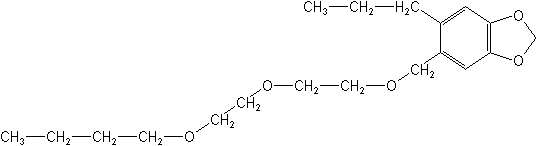
IUPAC5-[2-(2-butoxyethoxy)ethoxymethyl]-6-propyl-1,3-benzodioxole
or
2-(2-butoxyethoxy)ethyl 6-propylpiperonyl ether -
CAS5-[[2-(2-butoxyethoxy)ethoxy]methyl]-6-propyl-1,3-benzodioxole
-
CAS No.51-03-6
-
Molecular FormulaC19H30O5
-
Molecular Structure
-
Category
-
ActivitySynergist
-
PremixPIPERONYL BUTOXIDE+PYRETHRINS
Piperonyl butoxide
Permethrin+Piperonyl butoxide+tetramethrin
Permethrin+Piperonyl butoxide
Permethrin+bioallethrin+Piperonyl butoxide
Lambda-cyhalothrin+Piperonyl butoxide+tetramethrin
Lambda-cyhalothrin+chlorpyrifos+Piperonyl butoxide
Deltamethrin+s-bioallethrin+Piperonyl butoxide
Cypermethrin+Piperonyl butoxide+tetramethrin
Cypermethrin+Piperonyl butoxide
Bioallethrin+Piperonyl butoxide+tetramethrin
Allethrin+cyfluthrin+Piperonyl butoxide
Dust, emulsifiable concentrate, fogger, paper coating, pressurized spray, solution, wettable powder.
Premix Parters: 2,4-D; simazine sodium chlorate sodium metaborate;
-
Physical PropertiesMolecular weight:338.4; Physical form:Colourless liquid; ( tech. is a yellow oil). Density:1.060 (20 °C) (ENDQC-4 method); Composition:90% min. piperonyl butoxide. Flash point:140 °C (ASTM D93); Vapour pressure:2.0 × 10-2 mPa (60 °C) (gas saturation method); Henry constant:<2.3 × 10-6 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 4.75; Solubility:In water 14.3 mg/l (25 °C). Soluble in all common organic solvents including mineral oils and fluorinated aliphatic hydrocarbons (aerosol propellants).; Stability:Essentially stable to hydrolysis at pH 5, 7 and 9 in sterile buffers in the dark at 25 °C. Rapidly degraded in aqueous solution ( pH 7) in sunlight (DT50 8.4 h).
-
ToxicologyOral:Acute oral LD50 for rats and rabbits c. 7500 mg/ kg. Percutaneous:Acute percutaneous LD50 for rats >7950, rabbits 1880 mg/kg. Not irritant to eyes or skin; not a skin sensitiser. Inhalation: LC50 for rats >5.9 mg/l.
-
Environmental ProfileEcotoxicology:
Algae: EC50 (cell volume) for Chlorella fusca 44µmol/l (Pestic. Sci., 47, 337 (1996)).Bees: LD50 >25µg/bee.Birds:Acute oral LD50 for bobwhite quail >2250 mg/ kg.Daphnia: LC50 (24 h) 2.95 mg/l.Fish: LC50 (24 h) for carp 5.3 mg/l.
Environmental fate:
Animals:In mammals (and also in insects), oxidative attack on the carbon atom of the methylenedioxy group leads to the formation of the dihydroxyphenyl compound. Oxidative degradation of the side-chain also occurs. Elimination is as the glucoside or amino acid deSoil: DT50 for aerobic soil metabolism c. 14 d. Koc 399-830. Although mobile in sandy soil, it is not expected to leach under outdoor conditions, where rapid degradation occurs.WATER SOLUBILITY: 14.3 mg/l at 25°C -
Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide

 0
0 Subscribe
Subscribe