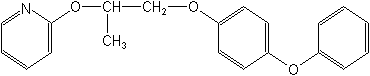-
Common NamePyriproxyfen
-
中文通用名吡丙醚
-
IUPAC4-phenoxyphenyl (RS)-2-(2-pyridyloxy)propyl ether
-
CAS2-[1-methyl-2-(4-phenoxyphenoxy)ethoxy]pyridine
-
CAS No.95737-68-1
-
Molecular FormulaC20H19NO3
-
Molecular Structure
-
Category
-
ActivityInsect Growth Regulator.Pyriproxyfen has activity against eggs and juveniles of the orders Diptera, Heteroptera, Lepidoptera and Thysanoptera. It is active by contact and ingestion. The product has residual activity lasting up to 35 days although this can be reduced by rainfall immediately after application. Adult females treated with the compound produce non-viable eggs. This effect has been shown to last for up to 14 days after treatment. Pyriproxyfen has translaminar activity which enhances its ovicidal and transovarian activity. The activity against late larval instars can be weaker than the ovicidal activity. In this case, the product is recommended for use in combination with, or in sequence with, other insecticides.
Pyriproxyfen has low toxicity to beneficial insects. Sumitomo recommends its use in IPM and IRM programmes.
Field trials in South Korea (J. Vector Ecol.) showed that a 0.5% granular formulation of pyriproxyfen, applied at 0.05-1.0 mg/L, was effective for the control of the mosquito, Aedes togoi, for a period of 40 days. Earlier studies (Jpn. J. Sanit. Zool.) had shown good control of Culex pipiens and C tritaeniorhynchus.
A monitoring program conducted in Arizona (J Econ Entomol, 2003) from 1996, the year of registration of pyriproxyfen on Arizona cotton, to 1999 detected high susceptibility to the insecticide among whiteflies (Bemisia argentifolii) in 1996. No resistance was observed also in 1997 and 1998 but a drop in whitefly mortality and reduced susceptibility to the product were first detected in 1999, possibly indicating the first signs of resistance development against the insecticide.
In one particular study (IPPC, 2004), neonicotinoids and insect growth regulators such as pyriproxyfen were tested on strawberries under greenhouse and field conditions. Results showed significant activity against whiteflies. -
CropUseCropUses:
acerola, almonds, apples, aubergines, blueberries, bushberries, citrus, cotton, courgettes, cucumbers, feijoa, grapefruits, green peppers, greenhouse ornamentals, guava, jaboticaba, juneberry lemons, limes, lingonberry, longan, lychees, olives, oranges, ornamentals, passion fruit, pears, peppers, persimmons, plums, pulasan, rambutan, salal, starfruit, stone fruits, strawberries, tomatoes, tree nuts, tropical fruit, fruiting vegetables, walnuts, wax jambu, animal health, public health
Cotton: 50-100 g ai/ha
-
PremixSpirotetramat+Pyriproxyfen
Pyriproxyfen+Thiamethoxam
Pyriproxyfen+Dinotefuran
Pyriproxyfen+Buprofezin
Pymetrozine+Pyriproxyfen
Flonicamid+Pyriproxyfen
Emamectin benzoate+Pyriproxyfen
-
Physical PropertiesMolecular weight:321.5; Physical form:Colourless crystals; ( tech. is a pale yellow, waxy solid, with a faint odour). Density:1.24 (25 °C); Melting point:45°-47°C; Flash point:119 °C (Pensky-Martens closed test); Vapour pressure:<0.013 mPa (23 °C); Partition coefficient(n-octanol and water):logP = 5.37; Solubility:In hexane 400, methanol 200, xylene 500 (all in g/ kg, 20-25 °C).
-
Toxicology(Rat): Acute Oral LD50 >5000 mg/kg; Inhalation LC50 (4 h) >1300 mg/m3. (Rabbit): Dermal LD50 >2000 mg/kg. Nonirritating to skin, eye.Percutaneous:Acute percutaneous LD50for rats >2000 mg/kg. ADI:0.009 mg/ kg.
-
Environmental ProfileWATER SOLUBILITY: Dispersible in water
Mallard duck
LD50 >2,000 mg/kg Virginia quail LD50>2,000 mg/kg
Mallard duck
LC50 >5,200 ppm Virginia quail LC50 >5,200 ppm
Rainbow trout [96 h]
LC50 >0,325 mg/L
Toad [96 h]
LC50 >0,270 mg/L
Daphnia [48 h]
EC50 >0,400 mg/L
Green algae [72 h]
EC50 >0,064 mg/L
Bees [contact 96 h]
LD50 >100mg/bee
Earthworm [14 d]
LD50 >1,000mg/kg soil
Fate in :
Pyriproxyfen is moderately volatile with a low water solubility but does not readily adsorb onto soil surfaces. Measured residue concentrations have been reported to decline by 50% in 24 hours in treated ponds. Pyriproxyfen adsorbs onto suspended organic matter and remains biologically activity for up to two months after an initial application. Its persistence in water in the absence of organic matter declines with increasing temperature and sunlight exposure.
Field studies conducted in California to evaluate the mobility and persistence of pyriproxyfen when applied to bare ground showed that both the parent compound and its major metabolites were found to be stable in soil for up to a year. The greatest pyriproxyfen concentrations were found in the 0-6 inch soil-layer. No pyriproxyfen residues were found above the level of quantitation below 12 inches. The half-life of pyriproxyfen in the 0-6 inch soil-layer was 36 days. No metabolite residues were found below 2 inches or greater than 120 days. The metabolism of 14C-Pyriproxyfen in a California sandy loam soil under aerobic conditions suggests that pyriproxyfen degrades rapidly via biological catalysis, having a half-life of 6.4 to 9.0 days and serving as a carbon source for soil microorganisms. The aerobic aquatic metabolic half-life of pyriproxyfen was 16.2 to 20.8 days. Metabolism in aerobic aquatic media proceeds through oxidative cleavage of the ester linkages. Pyriproxyfen degraded quickly under aerobic aquatic conditions (lake sediment and water) to two major metabolites (PYPAC and 4'-OH-Pyr), several minor intermediate metabolites, bound residues, and CO2.
Crustaceans and aquatic insect larvae are sensitive to pyriproxyfen, although adverse effects were found to be reversible. Pyriproxyfen did not affect mayfly, dragonfly, ostracods, cladocerans, copepods, or beetles. Planktonic organisms showed no significant adverse effects resulting from 0.01 ppm. Pyriproxyfen produced LC50 values of 0.098 ppm against the estuarine shrimp L. tenuicornis, about 12 times the estimated field concentration. Against non-target terrestrial organisms, pyriproxyfen produced severe deformities at molt of several predatory bugs. Pyriproxyfen is, however, practically non-toxic to bees. Bumblebee colonies were found to develop normally after feeding on pyriproxyfen-sucrose solution. -
Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)
Porduct NewsMore
Canada to approve continued use of pyriproxyfen
Cropchem’s Taura 200 EC registered against whitefly in Brazil
Canada to re-evaluate pyriproxyfen
Sumitomo Chemical develops mosquito larvicide SumiLarv® 2 MR
Related CompaniesMore
Rudong Zhongyi Chemical Co., Ltd.
Country: China
Pyraclostrobin Trifloxystrobin Prothioconazole tebuconazole pyriproxyfen cyproconazole cyazofamid famoxadone
Country: India
Oxyfluorfen Kasugamycin Carfentrazone-ethyl Bispyribac-sodium Pyrithiobac-sodium Quizalofop-P-ethyl Halosulfuron-methyl Carbendazim+mancozeb Tricyclazole Flusilazole Flusilazole+Carbendazim Iprovalicarb+propineb Propineb Difenoconazole+Propiconazole Isoprothiolane Kasugamycin+copper oxychloride Azoxystrobin+difenoconazole Azoxystrobin+Tebuconazole Propargite Cartap hydrochloride Bifenthrin Carbosulfan Emamectin benzoate Acetamiprid Buprofezin Spinosad Chlorantraniliprole Chlorantraniliprole Spiromesifen Imidacloprid Flubendiamide Cyflumetofen Pyriproxyfen Cartap hydrochloride Pendimethalin Pendimethalin Imazethapyr Clodinafop-propargyl Paraquat dichloride Chlorimuron-ethyl 2,4-D dimethyl amine salt Butachlor Copper oxychloride Carbendazim Copper hydroxide Hexaconazole Kasugamycin Carbendazim+mancozeb Carboxin+thiram Carboxin+thiram Propiconazole Imidacloprid Cartap hydrochloride Cartap hydrochloride Sulfoxaflor Fipronil Lambda-cyhalothrin Lambda-cyhalothrin Quinalphos Phenthoate Chlorpyrifos Dinotefuran Fipronil Fipronil Triazophos Monocrotophos Cypermethrin Cypermethrin Chlorantraniliprole Lambda-cyhalothrin Diafenthiuron Chlorpyrifos+cypermethrin
Nantong Shizhuang Chemical Co.,Ltd.
Country: China
Indoxacarb Dazomet Pyriproxyfen Pymetrozine
Country: China
Imidacloprid Pendimethalin Paraquat Pyrazosulfuron-ethyl+pretilachlor Pyraclostrobin+metiram Pirimiphos-methyl Lambda-cyhalothrin Glufosinate-ammonium Propanil Bispyribac-sodium Pyriproxyfen Trifluralin
Shenzhen Baocheng Chemical Industry CO.,LTD.
Country: China
Glyphosate Emamectin benzoate Lambda-cyhalothrin Pyraclostrobin Bromacil Glufosinate-ammonium Tembotrione Prothioconazole Topramezone Chlorantraniliprole

 0
0 Subscribe
Subscribe