-
Common NameTebufenpyrad
-
中文通用名吡螨胺
-
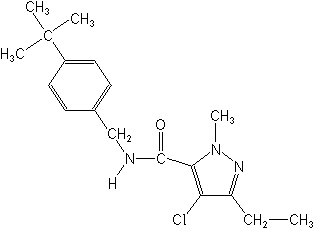
IUPACN-(4-tert-butylbenzyl)-4-chloro-3-ethyl-1-methylpyrazole-5-carboxamide
-
CAS4-chloro-N-[[4-(1,1-dimethylethyl)phenyl]methyl]-3-ethyl-1-methyl-1H-pyrazole-5-carboxamide
-
CAS No.119168-77-3
-
Molecular FormulaC18H24ClN3O
-
Molecular Structure
-
Category
-
ActivityAcaricide/Miticide
Tebufenpyrad is active against eggs and motile forms of mites. It has a rapid knockdown effect, with complete control of mites achieved within two days. It has no plant-systemic activity, but translaminar activity means that eggs and mites attached to the lower sides of leaves are controlled. Tebufenpyrad has residual activity lasting up to five weeks. The product also has secondary activity against hemipteran species. Its activity is not temperature dependent.
Tebufenpyrad inhibits electron transport during mitochondrial respiration at site 1 thus causing fatality.
In field trials, tebufenpyrad has shown no phytotoxicity at recommended rates. It is safe to beneficial insects. Mitsubishi recommends only one application per season in rotation with other acaricides to prevent the onset of resistance. -
CropUseCropUses:
Apples, aubergines, citrus, cotton, flowers, fruit, hops, melons, ornamentals, peaches, pears, peas, peppers, strawberries, tea, tomatoes, vegetables, vines, watermelons
Apples
10 g ai/hl; 500 g ai/ha
-
PremixEmulsifiable concentrate, emulsion oil in water, water dispersible granule, wettable powder.
Type
AI concn
Wettable powder (WP)
10% (w/w)
20% (w/w)
Emulsifiable concentrate (EC)
20% (w/v)
Oil-in-water emulsion (EW)
10% (w/v)
-
Physical PropertiesMolecular weight:333.9; Physical form:Colourless crystals. Density:1.0214; Melting point:61-62 °C; Vapour pressure:<1 × 10-2 mPa (25 °C); Henry constant:<1.25 × 10-3 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 5.04 (25 °C); Solubility:In water 2.8 mg/l (25 °C). Soluble in acetone, methanol, chloroform, acetonitrile, hexane, and benzene.; Stability:Stable to hydrolysis, DT50 >28 d ( pH 5, 7 and 9).;
-
ToxicologyOral:Acute oral LD50 for male rats 595, female rats 997, male mice 224, female mice 210 mg/kg. Percutaneous:Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin; slightly irritating to eyes (rabbits). Not a skin sensitiser (guinea pigs). Inhalation: LC50 for male rats 2660, female rats >3090 mg/m3. ADI:( JMPR ) 0.02 mg/kg b.w. [1996].
-
Environmental ProfileEcotoxicology:
Bees:Low toxicity to honeybees.Birds:Acute oral LD50 for mallard ducks >2000 mg/kg. Dietary LC50 (8 d) for mallard ducks and bobwhite quail >5000 mg/kg diet.Daphnia: LC50 (3 h) 1.2 mg/l.Fish: LC50 (48 h) for carp 0.073 mg/l.
Environmental fate:
Animals:Metabolite is N-[4-(1-hydroxymethyl-1-methylethyl)benzyl]-4-chloro-3-(1-hydroxyethyl)-1-methylpyrazole-5-carboxamide.Soil:Aerobic degradation occurs in soil, DT50 20-30 d. Koc 1380-4930.Plant:As for animals. -
Transport InformationHazard Class:III(Slightly hazardous)
Porduct NewsMore
More US registration reviews planned
Cyhalofop-butyl Diclofop-methyl Fluroxypyr Imazapic Noviflumuron Tebufenpyrad Etoxazole Polyoxin d zinc salt

 0
0 Subscribe
Subscribe