-
Common NameTriazoxide
-
中文通用名咪唑嗪
-
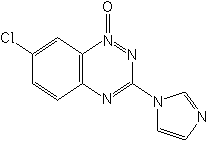
IUPAC7-chloro-3-imidazol-1-yl-1,2,4-benzotriazine 1-oxide
-
CAS7-chloro-3-(1H-imidazol-1-yl)-1,2,4-benzotriazine 1-oxide
-
CAS No.72459-58-6
-
Molecular FormulaC10H6ClN5O
-
Molecular Structure
-
Category
-
ActivityFungicide
-
PremixKresoxim-methyl+anthraquinone+fenpropimorph+triadimenol+triazoxide
Predominantly FS (flowable concentrates for seed treatment). Premix Parters: fenitrothion;
-
Physical PropertiesMolecular weight:247.7; Physical form:Light yellow crystals; ( tech., yellow-green crystals). Density:1.577 g/cm3 (22.5 °C); Melting point:182 °C; Vapour pressure:1 × 10-4 mPa (20 °C); 3 × 10-4 mPa (25 °C); Henry constant:7.3 × 10-7 Pa m3 mol-1 (20 °C); Partition coefficient(n-octanol and water):logP = 2.04 (23 °C); Solubility:In water 34 ( pH 7 and 9), 58 ( pH 4) (both in mg/l, 20 °C). In hexane 0.05, dichloromethane 32, isopropanol 1.8, toluene 6.9 (all in g/l, 20 °C).; Stability:Photolytically decomposed; DT50 (solid state) 1 d, (dissolved in water) 2-5 d.
-
ToxicologyOral:Acute oral LD50 for rats c. 150 mg/kg. Percutaneous:Acute percutaneous LD50 for rats >5000 mg/kg. Non-irritant to skin and eyes (rabbits). Inhalation: LC50 (4 h) for rats c. 0.75 mg/l (dust). Phytotoxicity:Shows good seed tolerance at recommended doses. ADI:( JMPR) 0.001 mg/kg b.w. [1993].
-
Environmental ProfileEcotoxicology:
Algae:ErC50 for Scenedesmus subspicatus 0.16 mg/l.Birds:Acute oral LD50 for Japanese quail 106-111 mg/kg. LC50 (5 d) for Japanese quail 3762, mallard ducklings 850 mg/kg diet.Daphnia: LC50 (48 h) 4.8 mg/l.Fish: LC50 (96 h) for golden orfe 7.4, rainbow trout 0.63 mg/l.Worms: LC50 for Eisenia foetida >1000 mg/kg dry soil.Other beneficial spp.:No effect on soil micro-organisms at 80 g/ha.
Environmental fate:
Animals:A complete absorption of triazoxide is followed by fast distribution in the body and rapid elimination; almost 100% of the dose is excreted within 48 h in the urine and faeces. Extensive metabolism of triazoxide was observed in the animal body (rat).Soil:Field studies in Germany revealed a medium fast dissipation rate from soil (average DT50 c. 2 mo). Low application rate (only 2-3 g a.i./dt seed) and low mobility in soilPlant:Because of its non-systemic properties, the amount of triazoxide taken up by plants after seed treatment is very low. Residues in the upper plant parts were never found. -
Transport InformationSignal Word:WARNING; Hazard Class:II(Moderately hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide

 0
0 Subscribe
Subscribe