-
Common NameTriclopyr
-
中文通用名三氯吡氧乙酸
-
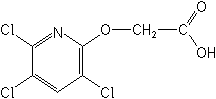
IUPAC3,5,6-trichloro-2-pyridyloxyacetic acid
-
CAS[(3,5,6-trichloro-2-pyridinyl)oxy]acetic acid
-
CAS No.55335-06-3
-
Molecular FormulaC7H4Cl3NO3
-
Molecular Structure
-
Category
-
ActivityHerbicide
-
PremixTriclopyr+clopyralid
Propanil+Triclopyr
Metsulfuron-methyl+triclopyr
Glyphosate+Triclopyr
Dicamba+Glyphosate+Triclopyr
Aminopyralid+Triclopyr-butotyl
Premix Parters: captan metalaxyl thiophanate-methyl; cyproconazole; fluoxastrobin prothioconazole; propiconazole; prothioconazole; tebuconazole; triadimefon;
-
Physical PropertiesMolecular weight:256.5; Physical form:Fluffy, colourless solid. Density:1.85 (21 °C).; Melting point:150.5 °C; Vapour pressure:0.2 mPa (25 °C, vapour pressure balance); Partition coefficient(n-octanol and water):logP = 0.42 (pH 5), -0.45 (pH 7), -0.96 (pH 9); pKa:3.97; Solubility:In water 0.408 (purified), 7.69 ( pH 5), 8.10 ( pH 7), 8.22 ( pH 9) (all in g/l, 20 °C). In acetone 581, acetonitrile 92.1, hexane 0.09, toluene 19.2, dichloromethane 24.9, methanol 665, ethyl acetate 271 (a; Stability:Stable under normal storage conditions and to hydrolysis, but subject to photodecomposition, DT50 <12 h.;
-
ToxicologyOral:Acute oral LD50 for male rats 692, female rats 577 mg/kg. Percutaneous:Acute percutaneous LD50 for rabbits >2000 mg/kg. Mild eye irritant; non-irritating to skin (rabbits). Inhalation: LC50 (4 h) for rats >256 ppm. Phytotoxicity:Clovers are sensitive, as also are larch, lodgepole pine, ornamentals, and edible crops.
-
Environmental ProfileEcotoxicology:
Algae: EC50 (5 d) for Selenastrum capricornutum 45 mg/l.Bees:Non-toxic to bees; contact LD50 >100 µg/bee.Birds:Acute oral LD50 for mallard ducks 1698 mg/kg. Dietary LC50 (8 d) for mallard ducks >5000, Japanese quail 3278, bobwhite quail 2935 mg/kg. Daphnia: LC50 (48 h) 133 mg/l.Fish:LC50 (96 h) for rainbow trout 117, bluegill sunfish 148 mg/l.
Environmental fate:
Animals:In mammals, following oral administration, excretion is primarily via the urine as the unchanged compound. For details of minor urinary metabolites, see C. Timchalk et al., Toxicology, 1990, 62, 71.Soil:In soil, fairly rapid degradation by microbial activity, with an average half-life of 46 d, depending on soil and climatic conditions. The major degradation product is 3,5,6-trichloro-2-pyridinol (which has a soil half-life of 30-90 d), with a smaller amoPlant:In plants, DT50 c. 3-10 d. The main metabolite is 3,5,6-trichloro-2-methoxypyridine. -
Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)
Porduct NewsMore
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
EU amended the conditions of use of herbicide triclopyr
Related CompaniesMore
Country: India
Permethrin Imidacloprid Cypermethrin Chlorpyrifos Alpha-cypermethrin Triclopyr Deltamethrin Fluroxypyr Quinalphos Temephos
Country: China
Oxyfluorfen Tebuconazole Metolachlor Azoxystrobin Clopyralid Triclopyr Tebuthiuron Acifluorfen Clethodim Imazapyr Picloram Pretilachlor Bifenthrin Amitraz Mesotrione
Country: China
Glufosinate-ammonium 2,4-D MCPA Dicamba Propanil Clethodim Glyphosate Captan Flumioxazin Sulfentrazone
Country: China
Fluroxypyr-meptyl Clodinafop-propargyl Glufosinate-ammonium Picloram Clopyralid Triclopyr-butotyl Flumioxazin Triclopyr Diquat Epoxiconazole
Jiangsu Fengshan Group Co., Ltd.
Country: China
Ethoprophos Imidacloprid Propargite Trifluralin Nicosulfuron Acetamiprid Chlorpyrifos Quizalofop-P-ethyl Cyhalofop-butyl Triclopyr

 0
0 Subscribe
Subscribe