-
Common NameFluthiacet-methyl
-
中文通用名嗪草酸甲酯
-
IUPACmethyl {2-chloro-4-fluoro-5-[(EZ)-5,6,7,8-tetrahydro-3-oxo-1H,3H-[1,3,4]thiadiazolo[3,4-a]pyridazin-1-ylideneamino]phenylthio}acetate
-
CASmethyl [[2-chloro-4-fluoro-5-[(tetrahydro-3-oxo-1H,3H-[1,3,4]thiadiazolo[3,4-a]pyridazin-1-ylidene)amino]phenyl]thio]acetate
-
CAS No.117337-19-6
-
Molecular FormulaC15H15ClFN3O3S2
-
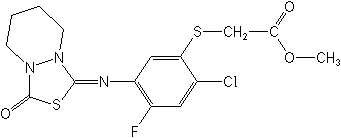
Molecular Structure
-
Category
-
ActivityHerbicide
Fluthiacet-methyl acts rapidly following application to the foliage of sensitive species. Symptoms of leaf necrosis and chlorosis are evident within 24-48 hours of treatment and death occurs within 3 days of application. This activity is light-dependent. Application can be carried out at a number of weed growth stages without affecting the levels of weed control and crop selectivity, although weeds taller than 10 cm may require split applications or mixtures to ensure effective control.
The product can be tank-mixed with standard herbicides to give a broader spectrum of control. Optimum activity is achieved when fluthiacet-methyl is used in conjunction with adjuvants such as crop oil concentrates, non-ionic surfactants and silicone additives. It has been suggested that fluthiacet-methyl is a pro-herbicide. The biologically active entity is the urazole form of the compound, formed by the action of glutathione-S-transferase on the parent isourazole.
The Public Interest Finding reported in the EPA fact sheet states that fluthiacet is effective at controlling certain broadleaf weeds that are common throughout soybean production areas, and is particularly effective at controlling velvetleaf. Due to lower use rates than the alternative herbicides, Pinnacle, Pursuit, Basagran and Resource that will be replaced, the total herbicide volume applied to soybeans would be reduced’.
In field trials, Novartis and Kumiai reported that fluthiacet-methyl showed complete selectivity to maize and soybeans and was safe to rotational crops. Fluthiacet-methyl gave superior control of weeds at much lower rates than commercially available herbicides. The maximum allowed seasonal application for fluthiacet-methyl is 10 g\ha.
Crop selectivity studies published in Weed Science (2000) showed that tolerance of maize to applications of fluthiacet-methyl was attributable to decreased uptake, retention and translocation in combination with increased plant metabolism. In soya, crop tolerance was attributed to decreased herbicide retention and increased metabolism. Of the weeds tested, the most tolerant was Amaranthus retroflexus and this was due to enhanced herbicide metabolism.
Field trials conducted in 1997 (Weed Technology) showed greatest control of velvetleaf (Abutilon theophrasti) when fluthiacet was applied post-emergence to weeds at 45-60 cm tall. When applied following a pre-em treatment with metolachlor, fluthiacet gave increased control compared with 2,4-D. In corn, velvetleaf control was greatest when fluthiacet was tank-mixed with nicosulfuron + dicamba or nicosulfuron + atrazine. In soybean, control of giant foxtail, lambsquarters, redroot pigweed and velvetleaf at 56 DAT was >85% with metolachlor + metribuzin (pre-em) followed by fluthiacet (post-em). Late post-em treatments with glyphosate or glyphosate + fluthiacet gave higher levels (>90%) of control. -
CropUseCropUses:
Soybeans, maize, cotton
Maize
3-5 g ai/ha
Cotton
5 g ai/ha
Soybeans
3-5 g ai/ha
-
PremixPyroxasulfone+fluthiacet-methyl
Fomesafen+fluthiacet-methyl
fluthiacet-methyl+mesotrione
-
Physical PropertiesMolecular weight:403.9; Physical form:White powder. Density:0.43 (bulk, 20 °C); Melting point:105.0-106.5 °C ( OECD 102); Vapour pressure:4.41 <×10-4 mPa (25 °C); Henry constant:2.1 × 10-4 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 3.77 (25 °C); Solubility:In water 0.85 (distilled), 0.78 ( pH 5 & 7), 0.22 ( pH 9) mg/l (25 °C). In methanol 4.41, acetone 101, toluene 84, n-octanol 1.86, acetonitrile 68.7, ethyl acetate 73.5, dichloromethane 9 (all in g; Stability:In water, DT50 484.8 d ( pH 5), 17.7 d ( pH 7), 0.2 d ( pH 9). In light, DT50 4.92 d.
-
ToxicologyOral:Acute oral LD50 for rats >5000 mg/ kg. Percutaneous:Acute percutaneous LD50 for rabbits >2000 mg/kg. Non-irritant to skin and slightly irritant to eyes (rabbits). Inhalation: LC50 (4 h, nose exposure) for rats >5.048 mg/l air.
-
Environmental Profile
Ecotoxicology:
Algae: EC50 for Selenastrum capricornutum 2.86 μg/l. NOEL (5 d) for Anabaena flos-aquae 18.4 μg/l.Bees: LD50 (contact) >100 <μg/bee.Birds:Acute oral LD50 for bobwhite quail and mallard ducks >2250 mg/kg. LC50 for blue quail >5620 ppm. Dietary LC50 (5 d) for bobwhite quail and mallard ducks >Daphnia: LC50 (48 h) >2.3 mg/l.Fish: LC50 (96 h) for trout 0.043, carp 0.60, bluegill sunfish 0.14, sheepshead minnow 0.16 mg/l.Worms: LC50 for earthworms >948 mg/ kg dry soil.Other aquatic spp.: EC50 (96 h) for Eastern oyster 700, mysid shrimp 280 ppb. EC50 for Lemna gibba 2.2 ppb.
Environmental fate:
Animals:In the rat, within 48 h, 80% is eliminated via the faeces, 14% via urine. Metabolism proceeds via hydrolysis of the methyl ester, isomerisation at the thiadiazole ring and hydroxylation of the tetrahydropyridazine moiety.Soil: DT50 (hydrolysis, pH 7) 18 d, (photolysis on soil) 21 d, ( u.v. light) 2 h. In loam soil, DT50 1.2 d (25 °C, 75% of max. water capacity). Koc Plant:Field residues in beans are >0.01 ppm. In glasshouse studies, negligible residues are found at 10 ×?rate; organosoluble metabolites are similar to those in the rat. -
Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)

 0
0 Subscribe
Subscribe