-
Common NameVinclozolin
-
中文通用名乙烯菌核利
-
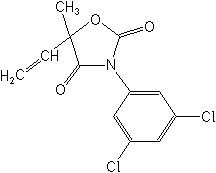
IUPAC(RS)-3-(3,5-dichlorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
-
CAS3-(3,5-dichlorophenyl)-5-ethenyl-5-methyl-2,4-oxazolidinedione
-
CAS No.50471-44-8
-
Molecular FormulaC12H9Cl2NO3
-
Molecular Structure
-
Category
-
ActivityFungicide
-
PremixChlorothalonil+vinclozolin
Dry flowable, water dispersible granule, flowable, wettable powder in water soluble bags.
PREMIX PARTNERS:Carbendazim; Chlorothalonil; Thiophanate-methyl; Thiram
-
Physical PropertiesMolecular weight:286.1g/mol; Physical form:Colourless crystals, with a slight aromatic odour. Density:1.51; Composition:Tech. is 96% m/m pure. Melting point:108℃ (tech.); Vapour pressure:0.13 mPa (20℃); Partition coefficient(n-octanol and water):logP=3 (pH7); Solubility:In water 2.6 mg/l (20℃). In methanol 1.54, acetone 33.4, ethyl acetate 23.3, n-heptane 0.45, toluene 10.9, dichloromethane 47.5 (all in g/100 ml soln., 20℃).Stability:Stable up to 50 ℃. Stable for 24 h in acidic media. In 0.1N sodium hydroxide, 50% hydrolysis occurs in 3.8 h.
-
ToxicologyOral Acute oral LD50 for rats and mice >15 000, guinea pigs c. 8000 mg/kg.Skin and eye Acute percutaneous LD50 for rats >5000 mg/kg.Inhalation LC50 (4 h) for rats >29.1 mg/l air.
-
Environmental Profile
ECOTOXICOLOGY
Birds Acute oral LD50 for quail >2510 mg/kg. LC50 for quail >5620 mg/kg.Fish LC50 (96 h) for trout 22-32, guppies 32.5, bluegill sunfish 50 mg/l.Daphnia LC50 (48 h) 4.0 mg/l.Bees Not toxic to bees. LD50 >200 mg/bee.Worms Not toxic to earthworms.
ENVIRONMENTAL FATE
Animals In the hen, the major metabolic routes are epoxidation of the vinyl group, followed by hydration of the intermediate epoxide, and by hydrolytic cleavage of the heterocyclic ring (G. M. Dean et al., Proc. Br. Crop Prot. Conf. - Pests Dis., 1988, 2, 693-698). In rats, following oral administration, eliminated in approximately equal proportions in the urine and faeces, with the principal metabolite being N-(3,5-dichlorophenyl)-2-methyl-2,3,4-trihydroxybutanamide. Plants In plants, the primary metabolites are (1-carboxy-1-methyl)allyl 3,5-dichlorophenylcarbamate and N-(3,5-dichlorophenyl)-2-hydroxy-2-methyl-3-butenamide. Alkaline hydrolysis leads to loss of 3,5-dichloroaniline from vinclozolin and its metabolites. The metabolites exist as conjugates.Soil/Environment Metabolism occurs by loss of the vinyl group, cleavage of the 5-membered ring and eventual formation of 3,5-dichloroaniline. Koc 100-735. Soil degradation of vinclozolin takes place with half-lives of several weeks, and mainly leads to the formation of bound residues. -
Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide

 0
0 Subscribe
Subscribe