-
Common NameButralin
-
中文通用名仲丁灵
-
IUPAC(RS)-N-sec-butyl-4-tert-butyl-2,6-dinitroaniline
-
CAS4-(1,1-dimethylethyl)-N-(1-methylpropyl)-2,6-dinitrobenzenamine
-
CAS No.33629-47-9
-
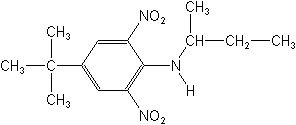
Molecular FormulaC14H21N3O4
-
Molecular Structure
-
Category
-
ActivityHerbicide, plant growth regulator
-
PremixOxadiazon+Butralin
Mesotrione+Butralin
Butralin+Diuron
Bensulfuron-methyl+Butralin
Emulsifiable concentrate. Premix Parters: MSMA sodium cacodylate; sodium cacodylate;
-
Physical PropertiesMolecular weight:295.3; Physical form:Yellow-orange crystals with a slightly aromatic odour. Density:1.063 (25 °C); Composition:Tech. is ×98%. Melting point:61 °C; ( tech., 59 °C); Vapour pressure:0.77 mPa (25 °C); Henry constant:7.58 × 10-1 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 4.93; Solubility:In water 0.3 mg/l (25 °C). In methanol 97.5, hexane 300 (both in g/l, 25 °C).; Stability:Decomposes at 265 °C. Hydrolytically and photochemically stable. Concentrates are stable on storage under dry conditions >3 y, but should not be stored <-5 °C or allowed to freeze.;
-
ToxicologyOral:Acute oral LD50 for male rats 1170, female rats 1049 mg/kg( tech.). Percutaneous:Acute percutaneous LD50 ( tech.) for rabbits ×2000 mg/kg. Slightly irritating to skin, moderately irritating to eyes (rabbits). Moderate skin sensitiser (Kligman & Magnusson test); not Inhalation: LC50 for rats >9.35 mg/l air.
-
Environmental ProfileEcotoxicology:
Algae: EC50 (5 d) for Selenastrum capricornutum 0.12 mg/l.Bees:Slightly toxic to bees.Birds:Acute oral LD50 for bobwhite quail >>2250, Japanese quail >>5000 mg/kg. Dietary LC50 (8 d) for bobwhite quail and mallard ducks >10 000 mg/kg diet.Daphnia: EC50 (48 h) 0.12 mg/l.Fish:LC50 (96 h) for bluegill sunfish 1.0, rainbow trout 0.37 mg/l.Other beneficial spp.:Not toxic to soil microflora at usual application rate. Application at 2-10 ×recommended rate did not significantly alter microbial population.
Environmental fate:
Animals:Extensively metabolised and excreted in urine and faeces. Metabolised in the rat by the primary metabolic processes of N-dealkylation, oxidation and nitro reduction, and by secondary processes of N-acetyl and glucuronic acid conjugation.Soil:In soil, microbial degradation occurs, with formation of the corresponding aniline, ring splitting and evolution of CO2 (P. C. Kearney et al., J. Agric. Food Chem., 1974, 22, 856). Persists in soil forPlant:In plants, metabolism leads to evolution of CO2. -
Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide
Related CompaniesMore
Zhangye Dagong Pesticide Chemistry Co., Ltd.
Country: China
Mesotrione Flumetralin Brassinolide Butralin Trifluralin Glyphosate

 0
0 Subscribe
Subscribe