-
Common NameFlumiclorac-pentyl
-
中文通用名氟烯草酸
-
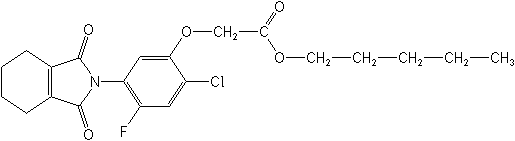
IUPACpentyl [2-chloro-5-(cyclohex-1-ene-1,2-dicarboximido)-4-fluororophenoxy]acetate
-
CASpentyl [2-chloro-4-fluoro-5-(1,3,4,5,6,7-hexahydro-1,3-dioxo-2H-isoindol-2-yl)phenoxy]acetate
-
CAS No.87546-18-7
-
Molecular FormulaC21H23ClFNO5
-
Molecular Structure
-
Category
-
ActivityHerbicide
Flumiclorac-pentyl is absorbed rapidly by foliage resulting in wilting, chlorosis and necrosis of the leaves within 24 hours if applied in bright sunlight. Optimum activity is obtained when the product is applied early post-emergence (2-4 leaf stage in weeds). Flumiclorac-pentyl is rainfast one hour after application. The short soil half-life means it poses no risk to rotation crops.
In field trials, flumiclorac-pentyl gave similar control to fomesafen, but at very much lower application rates. The yield of soybeans was also similar to fomesafen-treated crops.
Maize and soybean show good tolerance to flumiclorac as does the weed Brassica kaber. The tolerance of Brassica to this herbicide is attributed to enhanced herbicide metabolism compared to susceptible species. Increased metabolism plus decreased herbicide retention, absorbtion and translocation explain the selectivity in maize whereas the tolerance of soybean is due to decreased herbicide retention and increased herbicide metabolism (Weed Science, 2000). -
CropUseCropUses:
Soybeans, maize
30-90 g ai/ha
-
Physical PropertiesMolecular weight:423.9; Physical form:Beige solid, with a halide odour. Density:1.33 g/ml (20 °C); Melting point:88.9-90.1 °C; Flash point:68 °C; Vapour pressure:<0.01 mPa (22.4 °C); Partition coefficient(n-octanol and water):logP = 4.99 (20 °C); Solubility:In water 0.189 mg/l (25 °C). In methanol 47.8, hexane 3.28, n-octanol 16.0, acetone 590 (all in g/l).; Stability:Hydrolytic DT50 4.2 d ( pH 5), 19 h ( pH 7), 6 min ( pH 9). Aqueous photolysis DT50 are similar.
-
ToxicologyOral:Acute oral LD50 for rats 3.69 kg 0.86EC/ kg. Percutaneous:Acute percutaneous LD50 for rabbits >2.0 g 0.86EC/kg. Moderately irritating to skin and eyes; not a skin sensitiser. Inhalation: LC50 for rats 5.51 mg 0.86EC/l.
-
Environmental ProfileEcotoxicology:
Bees: LD50 (contact) >196 μg/bee.Birds:Acute oral LD50 for bobwhite quail >2250 mg/kg. Dietary LC50 for bobwhite quail and mallard ducks >5620 ppm.Daphnia: LC50 (48 h) >38.0 mg/l.Fish: LC50 for rainbow trout 1.1, bluegill sunfish 13-21 mg/l.
Environmental fate:
Soil:Rapidly degraded in soil: DT50 0.48-4.4 d in loamy-sand soil ( pH 7); degradates have DT50 c. 2-30 d. The a.i. is immobile in soil; degradates have low to mediumPlant:In soya beans and maize, the major metabolite is 2-chloro-4-fluoro-5-(4-hydroxy-1,2-cyclohexanedicarboximido)phenoxyacetic acid formed by reduction of the tetrahydrophthaloyl double bond and hydroxylation; other metabolic pathways include cleavage of the -
Transport InformationSignal Word:WARNING; Hazard Class:II(Moderately hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide

 0
0 Subscribe
Subscribe