-
Common NameBenthiavalicarb
-
中文通用名苯噻菌胺
-
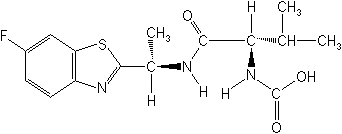
IUPACIsopropyl [(S)-1-[(R)-1-(6-fluorobenzothiazol-2-yl)ethylcarbamoyl]-2-methylpropyl] carbamate
-
CAS[S-(R, S)]-1-methylethyl [1-[[[1-(6-fluoro-2-benzothiazolyl)ethyl]amino]carbonyl]2-methylpropyl]carbamate
-
CAS No.413615-35-7
-
Molecular FormulaC18H24FN3O3S
-
Molecular Structure
-
Category
-
ActivityBenthiavalicarb is active against Oomycetes fungal plant pathogens. It exhibits fungicidal activity only against Oomycetes (except for Pythium spp) and not against Ascomycetes, Basidiomycetes and Deuteromycetes. Studies (Eur. J. Plant Path., 2003) show that benthiavalicarb is particularly effective for controlling downy mildew (Plasmopara viticola) in grapevines. The fungicide does not affect zoospore discharge from sporangia of P viticola, but strongly inhibits zoospore encystment, cystospore germination in vitro and mycelial growth, together with sporangial production in vivo.
Further studies (BCPC, 2003) show that against Phytophtora infestans, benthiavalicarb-isopropyl inhibited sporulation, germination of sporangia and cystspores at low concentration, but zoospore release and motility were not affected. Similar results were obtained in other trials with Plasmopara viticola, Pseudoperonospora cubensis and Peronospora parasitica.
Benthiavalicarb-isopropyl's precise mode of action is currently under investigation. The product is active against phenylamide resistant strains of Phytophtora infestansand strobilurin resistant strains of Pseudoperonospora cubensis,suggesting a different mode of action from the fungicides of these chemical groups. Benthiavalicarb-isopropyl does not affect respiration, synthesis of nucleic acid or protein or the function of the plasma membrane of P infestans.
Benthiavalicarb shows strong prophylactic and local activity in intact plants or detached leaves and low translaminar activity. The compound is not translocated from leaf to leaf in either a acropetal or basipetal direction.
Benthiavalicarb-isopropyl has strong preventive, curative and penetrant activities, excellent residual activity and rainfastness. It is recommended that benthiavalicarb-isopropyl should be used in combination with other contact fungicides in order to provide broad spectrum activities and resistance risk management. The effectiveness of benthiavalicarb makes it well suited for integration into a control programme against downy mildew disease in vineyards, and as a component to delay resistance build-up.
When applied at 1, 3 and 6 days post-inoculation, benthiavalicarb protected grapevines against downy mildew and inhibited sporulation of the pathogen. Similar results were obtained on leaf disks if benthiavalicarb was applied up to 96 h post-inoculation. Benthiavalicarb diminished the sporulation of P viticola when applied to established disease in the tissue. The product remained active on leaves for a period up to 28 days. Two foliar applications of benthiavalicarb, 2 weeks apart, to field-grown grapevines inhibited downy mildew development and were as effective as the standard metalaxyl-Cu treatment in controlling the disease. A formulated mixture of benthiavalicarb + Folpet was similar or superior in performance to metalaxyl-Cu and the new strobilurin trifloxystrobin in controlling downy mildew.
Benthiavalicarb-isopropyl alone at the rates of 25-75 g ai/ha and in combination with reduced rates of protectant fungicides was tested for the control of Phytophtora infestans, Plasmopara viticolaand Pseudoperonospora cubensis(BCPC 2003). Benthiavalicarb sprayed at 7-day intervals at the rates of 35-75 g ai/ha alone and in combination with mancozeb demonstrated excellent efficacy against potato late blight. The activity was equal to or surpassed that of the standard fungicides dimethomorph + mancozeb, mancozeb and fluazinam. Against vine downy mildew, excellent efficacy was achieved by spraying benthiavalicarb-isopropyl at 10-day intervals alone at the rate of 35-75 g ai/ha and in combination with folpet applied to both leaves and bunches. The activity equalled that of the standard fungicides dimethomorph + folpet and fosetyl A1 + folpet. Benthiavalicarb-isopropyl, both alone, at the concentration of 25-75 ppm, and in combination, proved to be highly effective against cucumber downy mildew by spraying at 7-day intervals. The activity surpassed that of standard fungicides chlorothalonil and metalaxyl + mancozeb.
Certis' new adjuvant, Zin Zan (1,2 bis(ethylhexyloxycarbonyl)ethanesulphonate) gained approval in the UK in 2006. Zin Zan improves the activity of Valbon against potato blight by increasing the rate and level of uptake of the active ingredients into the leaf. -
CropUseCropUses:
leafy vegetables, lettuce, potatoes, tobacco, tomatoes, vines
Potatoes
1.6 Kg/ha (benthiavalicarb + mancozeb)
Vines
0.5 Kg/ha (benthiavalicarb + folpet)
-
PremixBenthiavalicarb-isopropyl+mancozeb
Type
AI concn
Water dispersible granule (WG)
-
Toxicology
Acute oral (rat)
LD50 >5,000
-
Environmental Profile
Rainbow trout
LC50 >10 mg/L
Bobwhite quail
LD50 >2000 mg/kg
Bee [oral]
LD50 >100 μg/bee
Earthworm
LC50 >1000 mg/kg
Bluegill
LC50 >10 mg/L
Daphnia magna
LC50 >10 mg/L
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide

 0
0 Subscribe
Subscribe