-
Common NameDicofol
-
中文通用名三氯杀螨醇
-
IUPAC2,2,2-trichloro-1,1-bis(4-chlorophenyl)ethanol
or
α,α,α,4,4′-pentachloro-α-methylbenzhydryl alcohol -
CAS4-chloro-α-(4-chlorophenyl)-α-(trichloromethyl)benzenemethanol
-
CAS No.115-32-2
-
Molecular FormulaC14H9Cl5O
-
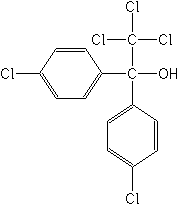
Molecular Structure
-
Category
-
ActivityAcaricide/Miticide
-
PremixFenpropathrin+dicofol
Emulsifiable concentrate, wettable powder. Premix Parters: calciferol.
-
Physical PropertiesMolecular weight:370.5. Physical form:Colourless solid; ( tech. is a brown, viscous oil), amber emulsion (41.1%). Specific gravity at 20°C, 1.130. At 20°C wt./gal. 9.4. Density:1.45 (25 °C; tech.); Composition:Tech. is 95% pure, composed of 80% dicofol and 20% its -1-(2-chlorophenyl)-1-(4-chlorophenyl)- isomer ('o,p'-dicofol'). Melting point:78.5-79.5 °C; Flash point:193 °C (open cup). Vapour pressure:0.053 mPa ( tech.). Partition coefficient(n-octanol and water):logP = 4.30; Solubility:In water 0.8 mg/l (25 °C). In acetone, ethyl acetate, toluene 400, methanol 36, hexane, isopropanol 30 (all in g/l, 25 °C). Stability:Stable to acids, but unstable in alkaline media, being hydrolysed to 4,4'-dichlorobenzophenone and chloroform; DT50 ( pH 5) 85 d, ( pH 7) 64-99 h, ( pH 9) 26 min.
-
ToxicologyOral:Acute oral
LD50 for male rats 595 mg/kg, female rats 578 mg/kg; rabbits 1810 mg/kg. Percutaneous:Acute percutaneous LD50 for rats >5000 mg/kg, rabbits >2500 mg/kg. Inhalation: LC50 (4 h) for rats >5 mg/l air. Phytotoxicity:Non-phytotoxic when used as directed, but aubergines and pears may be injured. ADI:0.05 mg/kg. -
Environmental ProfileEcotoxicology:
Algae:EC50 (96 h) for Scenedesmus 0.075 mg/l.Bees:Not toxic to bees; LD50 (contact) >50 µg tech./bee; (oral) >10 µg tech./bee.Birds: LC50 (5 d) for bobwhite quail 3010, Japanese quail 1418, ring-necked pheasants 2126, mallard ducks 1651 ppm. In eggshell quality and reproduction studies, NOAEL for American kestrel 2, mallard ducks 2.5, bobwhiDaphnia: LC50 (48 h) 0.14 mg/l.Fish: LC50 (96 h) for channel catfish 0.30 mg/l, bluegill sunfish 0.51 mg/l, largemouth bass 0.45 mg/l, fathead minnow 0.183 mg/l, sheepshead minnow 0.37 mg/l. LC50 (24 h) for rainbow trout 0.12 mg/l. Worms: 50 (7 d) 43.1 , (14 d) 24.6 ppm.Other aquatic spp.: LC50 (96 h) for mysid shrimp (Mysidopsis bahia) 0.06 mg/l; EC50 for oyster shell 0.15, fiddler crab 64, invertebrate early life stage (Hyalella) 0.19 mg/l.
Environmental fate:
Animals:In rats, following oral administration, 4,4'-dichlorobenzophenone and 2,2'-dichloro-1,1'-bis(chlorophenyl)ethanol are the principal metabolites. The same metabolites have been detected in laying hens and dairy goats.Soil:Soil photodegradationDT50 (silt loam) 30 d. Aqueous photodegradation DT50 ( pH 5, sensitised conditions) 1-4 d; (unsensitised conditions) 15-93 d. Soil metabolism (aerobic) in silt loaPlant:The principal metabolite in plants is 4,4'-dichlorobenzophenone.
WATER SOLUBILITY: 0.8 mg/l at 25° C. -
Transport InformationSignal Word:DANGER; Hazard Class:Ib (Highly hazardous).
Porduct NewsMore
Peru bans agrochemicals with active ingredient ‘dicofol’
Related CompaniesMore
Country: India

 0
0 Subscribe
Subscribe