-
Common NameDiflumetorim
-
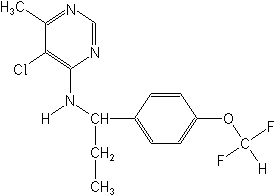
IUPAC(RS)-5-chloro-N-{1-[4-(difluoromethoxy)phenyl]propyl}-6-methylpyrimidin-4-ylamine
-
CAS5-chloro-N-[1-[4-(difluoromethoxy)phenyl]propyl]-6-methyl-4-pyrimidinamine
-
CAS No.130339-07-0
-
Molecular FormulaC15H16ClF2N3O
-
Molecular Structure
-
Category
-
ActivityFungicide.
Diflumetorim has protectant activity and should be applied before or in the early stages of infestation for white rust (Puccinia horiana) control. It also has some curative activity. The product inhibits fungal growth from germination to formation of conidiophores.
Diflumetorim is active against fungi resistant to sterol biosynthesis inhibitors, dithiocarbamates, benzimidazole, quinoxalines and antibiotics. It has shown no phytotoxicity to roses and chrysanthemums. -
CropUseCrop uses:
chrysanthemums, roses
Roses
5 g ai/hl
Chrysanthemums
10 g ai/hl
-
Physical PropertiesMolecular weight:327.8; Physical form:Pale yellow crystals. Density:0.490 (25 °C); Melting point:46.9-48.7 °C; Flash point:201.3 °C; Vapour pressure:3.21×10-1 mPa (25 °C); Henry constant:3.19×10-3 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 4.17 ( pH 6.86); pKa:4.5, weak base; Solubility:In water 33 mg/l (25 °C). Readily soluble in most organic solvents. Stability:Stable to hydrolysis at pH 4-9.
-
ToxicologyOral:
LD50 for male rats 448 mg/kg, female rats 534 mg/kg, male mice 468 mg/kg, female mice 387 mg/kg. Percutaneous:Acute percutaneous LD50 for male and female rats >2000 mg/kg. Slight skin and eye irritant (rabbits). Slight skin sensitisation (guinea pigs). Inhalation: LC50 for male and female rats 0.61 mg/l. ADI:0.26 mg/kg.?
-
Environmental ProfileEcotoxicology:?
Bees:LD50 (oral) >10 μg/bee; (contact) 29 μg/bee.Birds: LD50 for Japanese quail 881, mallard ducks 1979 mg/kg.Daphnia: LC50 (3 h) 0.96 mg/l.Fish: LC50 (48 h) for rainbow trout 0.025, carp 0.098 mg/l.?
Environmental fate:?
Soil:Soil dissipationDT50 (field, Japan) 60-100 d. Aerobic metabolism DT50 4.5 mo; the major metabolite, which barely exceeded 10% at any time, was diflumetorim hydroxylated at the pyrimid.
WATER SOLUBILITY: 33 mg/l at 25° C.
Mallard duck
LD50 1,979 mg/kg
Quail
LD50 881 mg/kg
Rainbow trout [48 h]
LC50 0.025 mg/L
Carp [48 h]
LC50 0.098 mg/L
Bee [contact]
LD50 29 μg/bee
Fate in soil:
In laboratory aerobic soil studies, diflumetorim is metabolised slowly and has a half-life of 4.5 months. 33-51% of the applied parent was mineralised to carbon dioxide after 44 weeks. The major metabolite (<10% of applied radioactivity) was diflumetorim hydroxylated at the 2-position on the pyrimidyl ring. Diflumetorim has low potential for leaching to ground water (Koc = 572-1710 on 4 Japanese soils). In field dissipation studies in Japan, the product has a DT50 of 60-100 days.Fate in aquatic systems:
Diflumetorim is stable to hydrolysis in water at pH 4 - 9. It is degraded photolytically in water with a DT50 of 151 hours in sterile water and 168 hours in river water. -
Transport InformationHazard Class:O (Obsolete as pesticide, not classified)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide

 0
0 Subscribe
Subscribe