-
Common NameEthaboxam
-
中文通用名噻唑菌胺
-
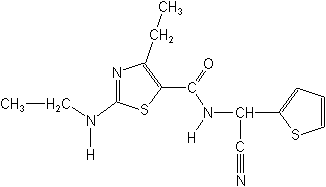
IUPAC(RS)-N-(α-cyano-2-thenyl)-4-ethyl-2-(ethylamino)-1,3-thiazole-5-carboxamide
-
CASN-(cyano-2-thienylmethyl)-4-ethyl-2-(ethylamino)-5-thiazolecarboxamide
-
CAS No.162650-77-3
-
Molecular FormulaC14H16N4OS2
-
Molecular Structure
-
Category
-
Activityethaboxam is a systemic fungicide which is taken into the plant and transported through the xylem following application to the soil. It is also transported translaminarly. The product has preventative, curative, systemic and residual activity. It inhibits penetration on the plant surface and is reported (BCPC 2002) to inhibit specifically mycelial growth and sporulation of P infestans. Research carried out in Korea has shown that ethaboxam weakly inhibits germination of zoospores or cysts. It is believed to have multiple sites of action as it inhibits migration of nuclei from the cyst to the growing germ tube and mycelia, and appears to inhibit oxygen consumption by mitochondria. Work is in progress to understand its mechanism of action more fully.
In growth chamber tests, ethaboxam completely inhibited mycelial growth of Phytophthora infestans at 0.1 - 0.5 mg/L, and P capsici at 1.0 mg/L. In terms of preventative activity, ethaboxam at 50 mg/L almost completely suppressed development of grape downy mildew, potato late blight, tomato late blight and red pepper blight; at 10 mg/L the extent of control was 100, 60, 94 and 95%, respectively. Ethaboxam was found to be persistent; 6 days after treatment the preventative activity on tomato late blight was 98% at 250 mg/l, 94% at 125 mg/L and 93% at 63 mg/L. To investigate its curative activity, zoospores of P infestans were inoculated 24 hours prior to the application of ethaboxam (250 mg/L); ethaboxam was found to be 38% effective whilst cymoxanil was 43% effective. In terms of translaminar activity, ethaboxam was 94% effective when applied on the lower surface and inoculation occurred on the upper surface, and 98% when applied on the upper surface and inoculation occurred on the lower surface. For systemic activity, ethaboxam was found to be 78% effective.
Trials in Korea found that ethaboxam alone and in combination with dimethomorph and with triflumizole was effective in controlling P capsici when applied either pre- or post-infection. There was no chemical damage to the crops.
LG Chemical recommends that LGC 30473 is applied every 10 days when there is a risk of disease infection, increasing to every seven days when disease pressure is high. Activity can be enhanced by combining the product with mancozeb. In field tests with 10% SC formulation (BCPC 2002), ethaboxam effectively controlled grape downy mildew on both leaves and bunches when used as a foliar spray applied at 100 - 250 g/ha at 7 - 10 day intervals. The optimum application rate for the control of potato late blight was found to be 200 g/ha at which high anti-sporulation and moderate systemic activities were observed. As a 25% WP formulation, ethaboxam was most effective against potato late blight at an application rate of 250 g/ha when applied to leaves at 7-10 day intervals. However, combinations are recommended for high disease pressure.
In field trials, LGC 30473 gave similar control to combination products containing metalaxyl and mancozeb. It is safe to crops and has shown no cross-resistance to phenylamide fungicides. It has proved effective against several strains of P infestans and P capsici resistant to phenylamide, and has not been observed to induce any mutant strains in laboratory studies.
An isolate of Pseudoperonospora cubensis which was resistant to kresoxim-methyl was found to be very sensitive to ethaboxam. Ethaboxam showed no inhibitory effect against Alternaria alternata, Botrytis cinerea, Cercospora betiicola, Diaporthe citri,Gibberella fujikuroi, Magnaporthe grisea, Penicillum italicum or Rhizoctonia solani. -
CropUseCropUses:
brassicas, cucurbits, fruiting vegetables, ginger, ginseng, grapes, leafy vegetables, legumes, lettuce, peppers, potatoes, ornamentals, sesame, tomatoes, vines, watermelon
Vines
250 g ai/hl
Vegetables
150-250 g ai/ha
Potatoes
250 g ai/ha
Cucurbits
150-200 g ai/hl
-
Premixethaboxam+clothianidin
-
Physical PropertiesMolecular weight:320.4; Pale yellow powder; no odor. Melting point: 185°C. Vapor pressure at 1.5 x 10-7 Pa at 25°C.
-
Toxicology(Rat): Oral LD50 >5000 mg/kg. (Mouse): Oral LD50 >2000 mg/kg
-
Environmental Profile
Ecotoxicology:
Rainbow trout [96 h]:LC50 = 2.0 mg/L;Bluegill sunfish [96 h]:LC50 >2.9 mg/L;Fathead minnow [96 h]:LC50 >4.6 mg/L;Daphnia [48 h]:EC50 = 0.33 mg/L;Algae [120 h]:EC50 >3.6 mg/L;Honeybee:LD50 >100 μg/bee;Earthworm:LD50 >1,000 ppm;Bobwhite quail:LD50 >5,000 mg/kg;
Environmental fate:
In soil: LGC 30437 has a soil half-life of 9 days.
In aquatic systems: LGC 30437 is decomposed in water by hydrolysis and photolysis. In aqueous hydrolysis, the half-life is dependent on pH and varies from 89 days at pH 4 to 46 days at pH 9. Under photolysis, the half-life is 16 days. -
Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)
Porduct NewsMore
US approved Valent’s Elumin™ Fungicide for Downy Mildew in Cucurbits
Canada approves Valent’s fungicide ethaboxam

 0
0 Subscribe
Subscribe