-
Common NameFamoxadone
-
中文通用名噁唑菌酮
-
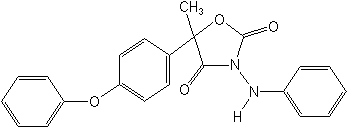
IUPAC(RS)-3-anilino-5-methyl-5-(4-phenoxyphenyl)-1,3-oxazolidine-2,4-dione
-
CAS5-methyl-5-(4-phenoxyphenyl)-3-(phenylamino)-2,4-oxazolidinedione
-
CAS No.131807-57-3
-
Molecular FormulaC22H18N2O4
-
Molecular Structure
-
Category
-
ActivityFamoxadone provides activity against a broad range of plant pathogens. It has protectant, translaminar and residual properties. In trials, famoxadone did not lose activity following simulated rainfall and its activity is termed ‘intracuticular’ by DuPont. The product was safe to target crops at twice the recommended use rate. DuPont recommends its use with established fungicides for season-long control.
The biochemical mode of action of famoxadone is inhibition of the mitochondrial respiratory chain at complex III resulting in decreased production of cellular ATP. In studies with Phytophthora infestans, application of famoxadone to the zoospores of this organism caused the cessation of oxygen consumption and motility within seconds, followed by the disintegration of the cells.
This product has been included in the new anti-resistance guidelines issued by the Strobilurin Type Action and Resistance (STAR) Working Group. The group has suggested that growers limit the number of applications to a maximum of four within a total fungicide spraying programme. DuPont points out that famoxadone is not a strobilurin and has no chemical relationship with these fungicides. Studies carried out by the company revealed that the risk of resistance to famoxadone-based mixtures is “very low”. However, famoxadone is generally classified as belonging to the ‘QoI’ group along with fenamidone and the strobilurins. Erysiphe graminis tritici and Sphaerotheca fuliginea have developed a resistance to this group due to a point mutation (G143A) in the cytochrome b gene, and were found to be as fit as sensitive wild-type under growth room environment conditions (BCPC, 2000). However, resistant populations of Plasmopora viticola (carrying the G143A mutation) were found to be less fit than the sensitive wild-type.
In Europe, famoxadone may be applied up to 12 times per season to grapes and up to 8 times per season on tomatoes. -
CropUseCropUses:
barley, cucurbits, lettuces, oilseed rape, peas, peppers, potatoes, sugarbeets, tomatoes, vines, wheat
Cereals
maximum dose, 280 g ai/ha
Vines, tomatoes
maximum dose, 90 g ai/ha
-
PremixMancozeb+famoxadone
Flusilazole+famoxadone
Famoxadone+Pyraclostrobin
Famoxadone+Oxathiapiprolin
Famoxadone+Fluazinam
Famoxadone+Dimethomorph
Famoxadone+Cyazofamid
Famoxadone+Azoxystrobin
Cymoxanil+famoxadone
Premix Parters:
cymoxanil; fosetyl-aluminum; mancozeb; propamocarb hydrochloride;
-
Physical PropertiesMolecular weight:374.4; Melting point:140.3-141.8 °C; Vapour pressure:6.4 × 10-4 mPa; Henry constant:4.61 × 10-3 Pa m3 mol-1 ( calc., 20 °C); Partition coefficient(n-octanol and water):logP = 4.65 ( pH 7); Solubility:In water 52 ppb (20 °C).;
-
ToxicologyOral:Acute oral LD50 for rats >5000 mg/ kg. Percutaneous:Acute percutaneous LD50 for rats >2000 mg/kg. Not a skin or eye irritant (rabbits). ADI:0.025 mg/ kg.
-
Environmental Profile
Ecotoxicology:
Bobwhite quail [8 d] LC50 >5,620 mg/kg ;Honeybee [oral] LD50 >63 μg/bee ;Mallard duck [8 d] LC50 >5,620 mg/kg;Honeybee [contact] LD50 >25 μg/bee;Rainbow trout [90 d] NOEC = 0.0014 mg/L;Daphnia[48 h] EC50 0.012 mg/L;Green algae [72 h] EbC50 = 0.022 mg/L;Earthworm LC50 = 470 mg/kg soil
Environmental fate:
Fate in :
Based on data submitted, famoxadone is acutely toxic to both fish and daphnia. Consequently, the EU SCP recommended the implementation of buffer zones to avoid toxicity to aquatic organisms.Fate in soil:
In laboratory studies carried out using various aerobic soil types at 20°C, the DT50 was found to vary between 2 and 11 days, and the DT90 between 56 and 248 days. In three soils, the major metabolites IN-KZ007 and IN-JS940 were found to have a DT50 of 1.5 to 10.3 days (mean 5.0 days), and 6 to 23 hours (mean 16 hours), respectively, at 20°C. In anaerobic soil at 20°C, the DT50 was 28 days whilst the DT90 was 91 days. Data from field studies was not required.
The Kd/Koc ratio in three soil types varied from 3300 to 4030 indicating that famoxadone is not very mobile. Column leaching studies were not required. In aged residue leaching studies, <0.1% of radioactivity was found in the leachate. The radioactivity was only detected in the top 0 - 5 cm of soil. Lysimeter/field leaching studies were not required.
Famoxadone has a photolytic half-life of 4.1 days.Fate in aquatic systems:
The rate of hydrolytic degradation at 20°C is pH-dependent with DT50 of 41 days at pH 5, 2 days at pH 7 and 1.5 hours at pH 9. Famoxadone is degraded photolytically with a half-life of 1.9 days in continuous light at pH 5.
In water/sediment study, the DT50 and DT90 in water are 0.07 - 0.43 hours and 1.6 - 23 hours, respectively, whilst the DT50 and DT90 for the whole system are 0.68 - 0.8 days and 14 - 15 days, respectively. -
Transport InformationHazard Class:O (Obsolete as pesticide, not classified)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide
Related CompaniesMore
Rudong Zhongyi Chemical Co., Ltd.
Country: China
Pyraclostrobin Trifloxystrobin Prothioconazole tebuconazole pyriproxyfen cyproconazole cyazofamid famoxadone
SINO AGRO-CHEMICAL INDUSTRY LTD.
Country: China
Prothioconazole Azoxystrobin Pyraclostrobin Methoxyfenozide Bifenazate Bispyribac-sodium Nicosulfuron Tebuconazole Pyribenzoxim Penoxsulam
Country: China
Clethodim Hexazinone Mesotrione Abamectin Acetamiprid Dimethoate Fipronil Imidacloprid Fosetyl-aluminium 2,4-D
Zhejiang Udragon Pesticides and Chemicals Co., Ltd.
Country: China
Boscalid Fludioxonil Metconazole Famoxadone Tetraconazole Trifloxystrobin Clodinafop-propargyl Mefenpyr-diethyl Difenoconazole
Country: China
2,4-D 2-ETHYLHEXYL ESTER florasulam+2,4-d 2-ethylhexyl ester Difenoconazole+Propiconazole Diflufenican+isoproturon Cymoxanil+famoxadone Fludioxonil+cyprodinil Flufenacet+diflufenican Glyphosate+2,4-D Imazamox+Bentazone Spiroxamine+Tebuconazole

 0
0 Subscribe
Subscribe