-
Common NameFenpiclonil
-
中文通用名拌种咯
-
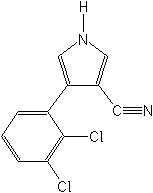
IUPAC4-(2,3-dichlorophenyl)-1H-pyrrole-3-carbonitrile
-
CAS4-(2,3-dichlorophenyl)-1H-pyrrole-3-carbonitrile
-
CAS No.74738-17-3
-
Molecular FormulaC11H6Cl2N2
-
Molecular Structure
-
Category
-
ActivityFungicide
Fenpiclonil has preventative activity and controls seed-borne diseases in cereals and tuber-borne diseases in potatoes. Fenpiclonil also has activity against soil-borne diseases and has residual activity lasting through to harvest. Novartis says that the treatment has no negative effects on seed germination. Potatoes can be treated post-harvest or pre-plant, using the dust formulation.
In field trials, fenpiclonil gave similar or better disease control to standard treatments, at lower application rates. -
CropUseCropUses:
canola, cereals, oilseed rape, potatoes, peas
cereals
200 g ai/tonne seed
potatoes
50 g ai/tonne seed
peas
200 g ai/tonne seed
-
Physical PropertiesMolecular weight:237.1; Physical form:White crystals. Density:1.53 (20 °C); Melting point:144.9-151.1 °C; Vapour pressure:1.1 × 10-2 mPa (25 °C); Henry constant:5.4 × 10-4 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 3.86 (25 °C); Solubility:In water 4.8 mg/l (25 °C). In ethanol 73, acetone 360, toluene 7.2, n-hexane 0.026, n-octanol 41 (all in g/l, 25 °C).; Stability:Not hydrolysed after 6 h at 100 °C between pH 3 and 9. Stable up to 250 °C.;
-
ToxicologyOral:Acute oral LD50 for rats, mice, and rabbits >5000 mg/ kg. Percutaneous:Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to eyes and skin (rabbits); non-sensitising (guinea pigs). Inhalation: LC50 (4 h) for rats >1.5 mg/l air. ADI:0.055 mg/ kg b.w.
-
Environmental ProfileEcotoxicology:
Algae: LC50 (5 d) for Scenedesmus subspicatus 0.22 mg/l.Bees:Non-toxic to honeybees; LD50 (oral and contact) >5 mg/bee.Birds:Acute oral LD50 for bobwhite quail >2510 mg/kg. LC50 for mallard ducks >5620, bobwhite quail 3976 ppm.Daphnia: LC50 (48 h) 1.3 mg/l.Fish: LC50 (96 h) for rainbow trout 0.8, carp 1.2, bluegill sunfish 0.76, catfish 1.3 mg/l.Worms: LC50 (14 d) for Eisenia foetida 67 mg/ kg soil.Other beneficial spp.:Harmless to carabid beetles.
Environmental fate:
Animals:Rapidly absorbed from the gastrointestinal tract into the general circulation; rapidly excreted and almost completely eliminated, mostly in the faeces. The dominant metabolic pathway is oxidation of the pyrrole ring at the 2-position. A minor pathway is hSoil:Relatively persistent in soil; formation of bound residues represents the major route for dissipation. In leaching and adsorption/desorption experiments, the compound proved to be immobile in soil, RMF 0.3. Photolytic DT50 in waPlant:Degradation proceeds via oxidation of the pyrrole ring followed by hydrolysis of the nitrile group. Opening of the pyrrole ring and hydroxylation of the phenyl ring are further degradation steps. The parent is, however, the relevant residue; between 13-15 -
Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide

 0
0 Subscribe
Subscribe