-
Common NameFenpropimorph
-
中文通用名丁苯吗啉
-
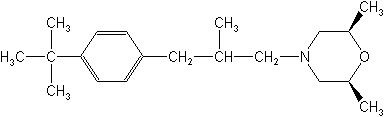
IUPACcis-4-[(RS)-3-(4-tert-butylphenyl)-2-methylpropyl]-2,6-dimethylmorpholine
-
CAS(2R,6S)-rel-4-[3-[4-(1,1-dimethylethyl)phenyl]-2-methylpropyl]-2,6-dimethylmorpholine
-
CAS No.67564-91-4
-
Molecular FormulaC20H33NO
-
Molecular Structure
-
Category
-
ActivityFungicide
-
PremixKresoxim-methyl+fenpropimorph
Kresoxim-methyl+epoxiconazole+fenpropimorph
Kresoxim-methyl+anthraquinone+fenpropimorph+triadimenol+triazoxide
Azoxystrobin+fenpropimorph
Emulsifiable concentrate, suspension concentrate.
Premix Parters: dimethoate; fenitrothion; monocrotophos;
-
Physical PropertiesMolecular weight:303.5; Physical form:Colourless, odourless oil ( tech., yellowish oil, with an aromatic odour). Density:0.933 (20 °C); Flash point:c. 105 °C (Pensky-Martens); 157 °C ( CIPAC MT12); Vapour pressure:3.5 mPa (20 °C); Henry constant:0.3 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 3.3 ( pH 5), 4.2 ( pH 7), 4.2 ( pH 9) (all at 22 °C); pKa:6.98 (20 °C), base; Solubility:In water 4.3 mg/l ( pH 7, 20 °C). In acetone, chloroform, ethyl acetate, cyclohexane, toluene, diethyl ether, ethanol >1 kg/ kg (20 °C).; Stability:Stable at room temperature in closed container for at least 3 years. Stable to light. Stable to hydrolysis at pH 3, 7 and 9 (50 °C).
-
ToxicologyOral:Acute oral LD50 for rats >3000 mg/ kg. Percutaneous:Acute percutaneous LD50 for rats >4000 mg/kg. Skin irritant; not an eye irritant (rabbits). No skin sensitisation (guinea pigs). Inhalation: LC50 (4 h) for rats >3580 mg/m3 air, with moderate irritation of the respiratory organs. ADI:0.005 mg/ kg b.w.
-
Environmental ProfileEcotoxicology:
Algae: EC50 (96 h) for Chlorella fusca 2.21 mg/l.Bees:Acute oral LD50 >100 μg/bee.Birds:Acute oral LD50 for mallard ducks >17 776, pheasants 3900 mg/kg. LC50 (5 d) for mallard ducks 5000, bobwhite quail >5000 mg/ kg.Daphnia: LC50 (48 h) 2.4 mg/l.Fish: LC50 (96 h) for rainbow trout 9.5, bluegill sunfish 3.2-4.6, carp 3.2 mg/l.Worms: LD50 (14 d) for earthworms 562 mg/ kg soil.Other aquatic spp.:EC10 (17 h) for Pseudomonas putida >1874 mg/l.Other beneficial spp.:Not dangerous to various beneficial insect species.
Environmental fate:
Animals:After oral administration to rats, fenpropimorph is rapidly absorbed and almost completely eliminated with urine and faeces. Residues in tissues were generally low.Soil:Degraded in soil by oxidation of the tertiary butyl group, in addition to oxidation and opening of the dimethylmorpholine ring. Half-life in soil ranges from 15 days in moderately humus loamy sand to 93 days in very humus loamy sand. Plant:Formation of polar metabolites due to cleavage of the morpholine ring and oxidation. -
Transport InformationHazard Class:III(Slightly hazardous)
Porduct NewsMore
BASF extends use of Versatilis for yellow sigatoka leaf spot on bananas
BASF launches Versatilis fungicides against Asian rust in Brazil
Related CompaniesMore
Country: China
Glufosinate-ammonium 2,4-D MCPA Dicamba Propanil Clethodim Glyphosate Captan Flumioxazin Sulfentrazone
Country: China
Acephate Pretilachlor Butachlor Niclosamide Fenclorim Fenpropimorph Difenoconazole Pyribenzoxim Metaldehyde Isoprothiolane

 0
0 Subscribe
Subscribe