-
Common NameAzoxystrobin
-
中文通用名嘧菌酯
-
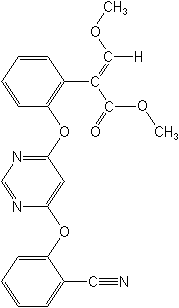
IUPACmethyl (E)-2-{2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl}-3-methoxyacrylate
-
CASmethyl (αE)-2-[[6-(2-cyanophenoxy)-4-pyrimidinyl]oxy]-α-(methoxymethylene)benzeneacetate
-
CAS No.131860-33-8
-
Molecular FormulaC22H17N3O5
-
Molecular Structure
-
Category
-
ActivityFungicide.
Azoxystrobin is a systemic fungicide, active against Ascomycetes, Basidiomycetes, Deuteromycetes and Oomycetes. It has preventative, curative and translaminar properties and residual activity lasting for up to eight weeks on cereals. The product demonstrates slow, steady foliar uptake and moves only in the xylem. Azoxystrobin inhibits mycelial growth and also has anti-sporulant activity. It is particularly effective at the early stages of fungal development (especially at spore germination) due to its inhibition of energy production. The product is classified as a Group K fungicide.
Azoxystrobin has been found to be very toxic to Macintosh apple trees and any apple varieties derived from Macintosh; phytotoxicity has also been observed on some crabapples.
The product can be mixed with Ambush, Bravo, Captan, copper hydroxide, Dominex, Dipel, Karate, Larvin, Fortress (D0059), Talstar and Thiodan.
Azoxystrobin effectively controlled damping-off and foliar diseases of vegetables (e.g. lettuce, tomatoes) and ornamentals (e.g. roses, carnations) in Northern Italy.
Field trials in Taiwan showed promising control of a wide range of diseases of winter peppers and a 10% increase in yield following two or three applications of Amistar (25% SC).
Strobilurins and the structurally unrelated famoxadone and fenamidone form a cross-resistance group called Qol-STAR by FRAC. Rapid resistance first developed in Erisyphe graminis tritici and Sphaerotheca fuliginea, and is attributed to the same mutation. In controlled environment studies, resistant strains of the former were as fit as sensitive populations, but resistant strains of the latter were significantly less fit than sensitive wild-types. Results with Venturia inaequalis suggested a different resistance mechanism.
Isolates of Alternaria solani (early blight) collected from potato fields in the USA before and after the introduction of azoxystrobin showed large increases in EC50 correlating with lack of field efficacy.
In US cotton, in-furrow application of azoxystrobin improved seedling survival and, generally, final yield. -
FormulationGR = Granule
SC = Suspension concentrate (=flowable concentrate)
WG = Water dispersible granules -
PremixTricyclazole+Azoxystrobin
Thifluzamide+Azoxystrobin
thiamethoxam+fludioxonil+azoxystrobin+mefenoxam
Thiamethoxam+azoxystrobin+fludioxonil+metalaxyl-M
Thiamethoxam+abamectin+azoxystrobin+fludioxonil+metalaxyl-M
Thiamethoxam+abamectin+azoxystrobin
Tetraconazole+Azoxystrobin
Spinosad+thiamethoxam+R-metalaxyl+fludioxonil+azoxystrobin
R-metalaxyl+fludioxonil+azoxystrobin+thiabendazole
Metalaxyl-M+Tebuconazole+Azoxystrobin
Metalaxyl-M+azoxystrobin
Meptyldinocap+Azoxystrobin
Mancozeb+Azoxystrobin
Jingangmycin A+Azoxystrobin
Isopyrazam+Azoxystrobin
Imazalil+Azoxystrobin
Hexaconazole+azoxystrobin
Flusilazole+Azoxystrobin
Fludioxonil+azoxystrobin+difenoconazole
Famoxadone+Azoxystrobin
EPOXICONAZOLE+AZOXYSTROBIN
Chlorothalonil+azoxystrobin
Boscalid+Azoxystrobin
benzovindiflupyr+Azoxystrobin
Azoxystrobin+Zinc thiazole
Azoxystrobin+Thiamethoxam+Thifluzamide
Azoxystrobin+Tebuconazole
Azoxystrobin+propiconazole
Azoxystrobin+Prochloraz+Thiamethoxam
Azoxystrobin+Ningnanmycin
Azoxystrobin+metalaxyl-M
Azoxystrobin+metalaxyl+thiophanate-methyl
Azoxystrobin+Metalaxyl+Tebuconazole
Azoxystrobin+metalaxyl
Azoxystrobin+Hymexazol
Azoxystrobin+Flutriafol
Azoxystrobin+flutolanil
Azoxystrobin+fludioxonil+metalaxyl-M+thiabendazole+thiamethoxam
Azoxystrobin+fludioxonil+metalaxyl-M+thiabendazole
Azoxystrobin+fludioxonil+metalaxyl-M
Azoxystrobin+fludioxonil
Azoxystrobin+fenpropimorph
Azoxystrobin+Dimethomorph
Azoxystrobin+difenoconazole
Azoxystrobin+cyproconazole
Azoxystrobin+cymoxanil
-
Physical PropertiesForm: White solid; Molecular weight: 403.4 g/mol;melting point 116℃.
-
Environmental ProfileEcotoxicology:
Bird: Oral LD50 >2000 mg/kg; Dietary (8 day) LC50 >5200 mg/kg (mallard duck, bobwhite quail). Bee: LD50 >200 μg/bee.
WATER SOLUBILITY:At 20℃: 6 mg/l.
Bobwhite quail
LD50 >2,000 mg/kg
Mallard duck
LD50 >2,000 mg/kg
Bee [contact/oral]
LD50 >200 μg/bee
Porduct NewsMore
Africa Watch: Imports of fungicides in African market on the rise
Mancozeb Chlorothalonil Azoxystrobin Pyraclostrobin Prochloraz
Atticus responds to 'meritless' patent infringement suit
UPL launched multisite fungicide Tridium in Argentina
Azoxystrobin Mancozeb Cyproconazole
Nufarm launches fungicide Tamiz in Brazil
Red Surcos prepares to launch first fungicide with nanotechnology in South America
FMC launches fungicide Rubric Max for soybean in Argentina
Sipcam Agro USA receives registration for fungicide Endow G
Use of Adama's fungicide Azimut extended to 20 more crops in Brazil
Azoxystrobin Azoxystrobin+Tebuconazole Tebuconazole
Rise in agricultural sustainability predicted to drive the global azoxystrobin market
EPA Approves Vive's Fenstro Foliar Combination Fungicide & Insecticide
Related CompaniesMore
SINO CHEMTECH (SHANGHAI) CO., LTD
Country: China
Tribenuron-methyl Nicosulfuron Dimethomorph Clodinafop-propargyl Diazinon Emamectin benzoate Imidacloprid Thiamethoxam Chlorpyrifos+cypermethrin Bromoxynil octanoate+MCPA-isooctyl BAAPE IR3535
Anhui Fengle Agrochemical Co., Ltd.
Country: China
Tribenuron-methyl Dimethomorph Bentazone Carboxin Quizalofop-P-ethyl Nicosulfuron Clomazone Propiconazole Chlorpyrifos Clopyralid Clodinafop-propargyl
Jiangsu Sword Agrochemicals Co., Ltd.
Country: China
Triadimefon Triadimenol Tebuconazole Diniconazole Bitertanol Cyproconazole Propineb Paclobutrazol Metribuzin Bentazone
Yancheng Limin Chemical Co.,Ltd
Country: China
Triadimefon Triadimenol Hexaconazole Flutriafol Imidacloprid Paclobutrazol Tebuconazole Thifluzamide Indoxacarb Uniconazole
Hebei Guanlong Agrochemical Co.,Ltd
Country: China
Thiram Ziram Captan Dimethomorph Flusilazole Acetamiprid Iprodione Difenoconazole Prochloraz Azoxystrobin

 0
0 Subscribe
Subscribe