-
Common NameImazaquin
-
中文通用名咪唑喹啉酸
-
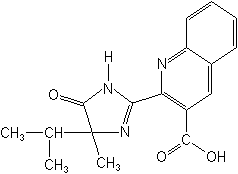
IUPAC2-[(RS)-4-isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl]quinoline-3-carboxylic acid
-
CAS2-[4,5-dihydro-4-methyl-4-(1-methylethyl)-5-oxo-1H-imidazol-2-yl]-3-quinolinecarboxylic acid
-
CAS No.81335-37-7
-
Molecular FormulaC17H17N3O3
-
Molecular Structure
-
Category
-
ActivityHerbicide
-
PremixLiquid concentrate; dispersible granule.
Premix Parters: acephate; bifenthrin; buprofezin; carbosulfan zeta-cypermethrin; carboxin metalaxyl; cyfluthrin; lambda-cyhalothrin; lambda-cyhalothrin pheromone; mancozeb; mancozeb thiophanate-methyl; metalaxyl; metalaxyl tebuconazole; pyridaben; thiram;
-
Physical PropertiesMolecular weight:311.3; Physical form:Tan solid, with slightly pungent odour. Composition:Tech. is >95% pure. Melting point:219-224 °C ( decomp.); Vapour pressure:<0.013 mPa (60 °C); Partition coefficient(n-octanol and water):logP = 0.34 (22 °C); pKa:3.8; Solubility:In water 60-120 mg/l (25 °C). In toluene 0.4, dimethylformamide 68, dimethyl sulfoxide 159, dichloromethane 14 (all in g/l, 25 °C).; Stability:Stable for 3 months at 45 °C, 2 y at room temperature when stored in the dark. Rapidly degraded on exposure to u.v. light.
-
ToxicologyOral:Acute oral LD50 for male and female rats >5000, female mice 2363 mg/ kg. Percutaneous:Acute percutaneous LD50 for male and female rabbits >2000 mg/kg. Non-irritating to eyes; mildly irritating to skin (rabbits). No skin sensitisation (guinea pigs). Inhalation: LC50 (4 h) for rats >5.7 mg/l air.
-
Environmental ProfileEcotoxicology:
Bees:Contact LD50 for honeybees >100 μg/bee.Birds:Acute oral LD50 for bobwhite quail and mallard ducks >2150 mg/kg. Dietary LC50 (8 d) for bobwhite quail and mallard ducks >5000 mg/ kg diet.Daphnia: LC50 (48 h) 280 mg/l.Fish: LC50 (96 h) for channel catfish 320, bluegill sunfish 410, rainbow trout 280 mg/l.
Environmental fate:
Animals:Imazaquin is metabolically inert in rats. Following oral administration, almost all was excreted in the urine as the unchanged compound within 2 days. No accumulation in blood or tissues.Soil:Steadily degraded in soil by microbial activity and photolysis, but may remain active in the soil for several weeks to several months, depending on environmental conditions (G. Basham et al., Weed Sci., 1987, 35,Plant:Rapidly metabolised by soya bean plants to inactive compounds, formed by opening of the imidazolinone ring at the ring amide (D. L. Shaner & P. A. Robson, Weed Sci., 1985, 33, 469). -
Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)
Porduct NewsMore
Sipcam's Coastal Herbicide registered in US
Agro-Kanesho acquires Off ® II Flowable business from BASF in Japan
Amvac licensed Scepter-based herbicide from BASF
Related CompaniesMore
Shandong Cynda Chemical Co., Ltd.
Country: China
Imazaquin Imazamox Carfentrazone-ethyl Sethoxydim Pyraflufen-ethyl Imazethapyr Imazapyr Imazameth Clomazone Dimethomorph Clethodim Bispyribac-sodium

 0
0 Subscribe
Subscribe