-
Common NameDimethachlor
-
中文通用名二甲草胺
-
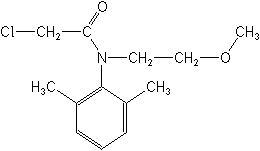
IUPAC2-chloro-N-(2-methoxyethyl)acet-2′,6′-xylidide
-
CAS2-chloro-N-(2,6-dimethylphenyl)-N-(2-methoxyethyl)acetamide
-
CAS No.50563-36-5
-
Molecular FormulaC13H18ClNO2
-
Molecular Structure
-
Category
-
ActivityHerbicide
-
PremixEmulsifiable concentrate. Premix Parters: atrazine.
-
Physical PropertiesMolecular weight:255.7; Physical form:Colourless crystals. Density:1.23 (20 °C); Melting point:45.8-46.7 °C; Vapour pressure:1.5 mPa (25 °C); Henry constant:1.7×10-4 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 2.17; Solubility:In water 2.3 g/l (25 °C). Readily soluble in most organic solvents, e.g. ketones, alcohols, chlorinated hydrocarbons, and aromatic hydrocarbons. In methanol, benzene, dichloromethane >800, n-octanol 440 (all in g/ kg, 25 °C).; Stability:Very stable in neutral media and in dilute acids. Hydrolysed in alkaline media ( pH 13). Readily soluble in most organic solvents, e.g. ketones, alcohols, chlorinated hydrocarbons, and aromatic hydrocarbons. In methanol, benzene, dichloromethane >800 g/kg, n-octanol 440 g/kg at 25°C.
-
ToxicologyOral:Acute oral
LD50 for rats 1600->2000 mg/kg. Percutaneous:Acute percutaneous LD50 for rats >3170 mg/kg. Slight irritant to skin and eyes of rabbits. Inhalation: LC50 (4 h) for rats >4450 mg/m3. Phytotoxicity:Non-phytotoxic to oilseed rape. ADI:0.001 mg/kg. -
Environmental ProfileEcotoxicology:
Algae:LC50 (72 h) for Scenedesmus subspicatus 0.053 mg/l.Bees: LD50 (oral and contact) >200 μg/bee.Birds: LC50 for mallard ducks 200 ppm, Japanese quail 524 ppm. Dietary toxicity (8 d) for mallard ducks >10 000 ppm.Daphnia: LC50 (48 h) 14.2-24 mg/l.Fish: LC50 (96 h) for bluegill sunfish 15, rainbow trout 3.9, crucian carp 8 mg/l.Worms: LC50 (14 d) 130 mg/kg.
Environmental fate:
Animals:The main metabolic reactions in rats are: O-dealkylation leading to O-desmethyl derivatives, substitution of the chlorine by a hydroxy group or by glutathione, reduction of the methylene chloride moiety giving rise to acetyl derivatives,Soil:Meanoc 63 ml/g. Slightly mobile in soil. In soil, 50 c. 4-15 d, DT90 <100 d; dissipation is by the formation of bound residues, and in parallel reactions, by successive oxidatiPlant:Metabolism involves conjugation of the chloracetyl group, hydrolysis and sugar conjugation at the ether group, and formation of a heterocyclic ether-keto ring.
WATER SOLUBILITY: 2.3 g/l at 25°C. -
Transport InformationSignal Word:CAUTION; Hazard Class:III (Slightly hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide

 0
0 Subscribe
Subscribe