-
Common NameFlucythrinate
-
中文通用名氟氰戊菊酯
-
IUPAC(RS)-α-cyano-3-phenoxybenzyl (S)-2-(4-difluoromethoxyphenyl)-3-methylbutyrate
-
CAScyano(3-phenoxyphenyl)methyl (αS)-4-(difluoromethoxy)-α-(1-methylethyl)benzeneacetate
-
CAS No.70124-77-5
-
Molecular FormulaC26H23F2NO4
-
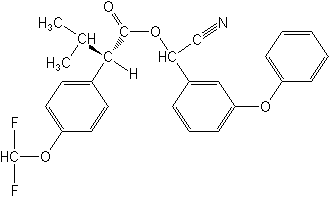
Molecular Structure
-
Category
-
ActivityInsecticide
-
CropUseapples, cabbage, field corn, head lettuce, and pears
-
FormulationWG = Water dispersible granules
WP = Wettable powder -
PremixFlucythrinate+fenitrothion
-
Physical PropertiesMolecular weight:451.4; Physical form:Dark amber, viscous liquid, with a faint ester-like odour ( tech.). Density:1.189 (22 °C); Vapour pressure:0.0012 mPa (25 °C); Henry constant:1.08 × 10-3 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 4.7 (25 °C); Solubility:In water 0.5 mg/l (21 °C). In acetone >820, xylene 1810, n-propanol >780, corn oil >560, cottonseed oil >300, soya bean oil >300, hexane 90 (all in g/l, 21 °C).; Stability:Rapidly hydrolysed in water under alkaline conditions, but more slowly under neutral or acidic conditions; DT50 (27 °C) c. 40 d ( pH 3), 52 d ( pH 5), 6.3 d ( pH 9).
-
ToxicologyOral:Acute oral LD50 for male rats 81, female rats 67, female mice 76 mg/ kg. Percutaneous:Acute percutaneous LD50 (24 h) for rabbits >1000 mg/kg. Non-irritating to skin and eyes (rabbits), but undiluted formulations may be irritating. No skin sensitisation. Inhalation: LC50 (4 h) for rats 4.85 mg/l air (aerosol). Phytotoxicity:Non-phytotoxic when used as directed. In certain treated crops, e.g. cotton and fruit trees, the green colouration of the foliage is i
-
Environmental ProfileEcotoxicology:
Bees:Toxic to bees, but also has a repellent effect. LD50 (topical, as a dust) 0.078 μg/bee. Birds:Acute oral LD50 for mallard ducks >2510, bobwhite quail 2708 mg/kg. Dietary LC50 (8 d) for mallard ducks 4885, bobwhite quail 3443 mg/ kg diet.Daphnia: LC50 (48 h) 0.0083 mg/l.Fish: LC50 (96 h) for bluegill sunfish 0.71, channel catfish 0.51, rainbow trout 0.32, sheepshead minnow 1.6 μg/l. Low insecticidal dosages and immobility in soils should minimise hazards to fish.
Environmental fate:
Animals:In rats, following oral administration, 60-70% is eliminated within 24 hours, and >95% within 8 days, in the faeces and urine. In the faeces, the parent compound makes up most of the material excreted, but in the urine and in tissue, several metabolites aSoil:In soil, there is low mobility and no leaching, with a half-life of c. 2 months. -
Transport InformationSignal Word:DANGER; Hazard Class:Ib(Highly hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide

 0
0 Subscribe
Subscribe