-
Common NameTetramethrin
-
中文通用名胺菊酯
-
IUPACcyclohex-1-ene-1,2-dicarboximidomethyl (1RS,3RS;1RS,3SR)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
or
cyclohex-1-ene-1,2-dicarboximidomethyl (1RS)-cis-trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
or
cyclohex-1-ene-1,2-dicarboximidomethyl (±)-cis-trans-chrysanthemate -
CAS(1,3,4,5,6,7-hexahydro-1,3-dioxo-2H-isoindol-2-yl)methyl 2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylate
-
CAS No.7696-12-0
-
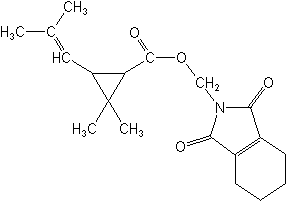
Molecular FormulaC19H25NO4
-
Molecular Structure
-
Category
-
Activity
Tetramethrin, the second generation of synthetic pyrethroids, is a contact insecticide with a rapid knockdown action on flies, mosquitoes, cockroaches, wasps and other insect pests in public, home and garden primarily for indoor pest control uses.
-
Pest Spectrumflies, mosquitoes, cockroaches, wasps and other insect pests.
-
FormulationAE = Aerosol dispenser
DP = Dustable powder
OL = Oil miscible liquide -
PremixPermethrin+tetramethrin
Permethrin+Piperonyl butoxide+tetramethrin
Lambda-cyhalothrin+Piperonyl butoxide+tetramethrin
CYPHENOTHRIN+TETRAMETHRIN
Cypermethrin+tetramethrin
Cypermethrin+Piperonyl butoxide+tetramethrin
Bioallethrin+Piperonyl butoxide+tetramethrin
-
Physical PropertiesMolecular weight:331.4; Physical form:Colourless crystals, with slight pyrethrum-like odour; ( tech. is a colourless to light yellow-brown solid). Density:1.1 (20 °C); Composition:Tech. is c. 92% pure. Melting point:68-70 °C; ( tech., 60-80 °C); Flash point:200 °C (Cleveland open cup); Vapour pressure:2.1 mPa (25 °C); Partition coefficient(n-octanol and water):logP = 4.6 (25 °C); Solubility:In water 1.83 mg/l (25 °C). In acetone, ethanol, methanol, hexane, n-octanol all >2 g/100 ml.; Stability:Sensitive to alkalis and strong acids; DT50 16-20 d ( pH 5), 1 d ( pH 7), <1 h ( pH 9). Stable on storage up to c. 50 °C. Stable in ketones, chloroform, xylene, common aer;
-
ToxicologyOral:Acute oral LD50 for rats >5000 mg/kg. Percutaneous:Acute percutaneous LD50 for rabbits >2000 mg/kg. Non-irritant to skin. Inhalation: LC50 (4 h) for rats >2.73 mg/l air.
-
Environmental ProfileEcotoxicology:
Bees:Toxic to bees.Birds:Acute oral LD50 for bobwhite quail >2250 mg/kg. Dietary LC50 for bobwhite quail and mallard ducks >5620 ppm.Daphnia: EC50 (48 h) 0.11 mg/l.Fish:LC50 (96 h) for rainbow trout 3.7, bluegill sunfish 16 µg/l.
Environmental fate:
EHC 98 notes rapid abiotic degradation in air and water, rapid metabolism and excretion in mammals, and no tendency to accumulate in tissues.Animals:In rats, following oral administration, around 95% of tetramethrin (metabolised) is eliminated in the urine and faeces within 5 days. The principal metabolite is 3-hydroxycyclohexan-1,2-dicarboximide (J. Miyamoto et al., J. Agric. BioSoil:Degradation involves cleavage of the ester bond, leading to chrysanthemic acid derivatives and phenoxybenzoic acid. These are further metabolised by hydroxylation and conjugation. -
Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)
Porduct NewsMore
Canada cancels certain uses of tetramethrin
Related CompaniesMore
Country: China
Clethodim Hexazinone Mesotrione Abamectin Acetamiprid Dimethoate Fipronil Imidacloprid Fosetyl-aluminium 2,4-D
Country: China
Brodifacoum Bromadiolone Difenacoum Chloralose Transfluthrin Tetramethrin Meperfluthrin Bioallethrin Dimefluthrin Prallethrin
Country: India

 0
0 Subscribe
Subscribe