-
Common NameResmethrin
-
中文通用名苄呋菊酯
-
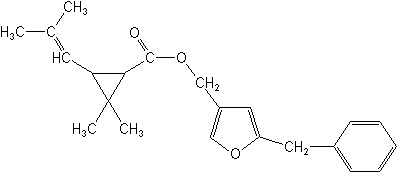
IUPAC5-benzyl-3-furylmethyl (1RS,3RS;1RS,3SR)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
or
5-benzyl-3-furylmethyl (1RS)-cis-trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
or
5-benzyl-3-furylmethyl (±)-cis-trans-chrysanthemate -
CAS[5-(phenylmethyl)-3-furanyl]methyl 2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylate
-
CAS No.10453-86-8
-
Molecular FormulaC22H26O3
-
Molecular Structure
-
Category
-
ActivityResmethrin kills insects by direct contact.3 Resmethrin is a Type I pyrethroid that affects the insects nervous system by interfering with sodium channels in the central and peripheral nervous system. Following exposure to resmethrin, sodium channels are kept open for prolonged periods of time, causing repetitive nerve discharge and increased excitation.
-
FormulationAE = Aerosol dispenser
EO = Emulsion ,water in oil
WP = Wettable powder
-
Physical PropertiesWaxy off-white to tan solid synthetic pyrethroid with characteristic chrysanthemate odor. Solubility 10% in kerosene; very soluble in xylene, methylene chloride, isopropyl alcohol and aromatic petroleum hydrocarbons.Molecular weight:338.4g/mol;Melting point:56.5 oC (pure (1RS)-trans- isomer);Boiling point: decomp>180℃;V.p. <0.01 mPa (25℃);KOWlogP = 5.43 (25℃);Henry <8.93×10-2Pa m3mol-1(calc.);S.g./density 0.958-0.968 (20℃); 1.035 (30℃);Solubility In water 37.9g/l (25℃). In acetone c. 30%, chloroform, dichloromethane, ethyl acetate, toluene >50%, xylene >40%, ethanol, n-octanol c. 6%, n-hexane c. 10%, isopropyl ether c. 25%, methanol c. 3% (all m/v, 20℃).;Stability Stable to heat and to oxidation. Decomposes rapidly on exposure to air and light (more slowly than pyrethrins) (J. H. Fales et al., CSMA Proc. 55th Annu. Meeting, December 1968; A. B. Hadaway et al., Bull. WHO, 1970, 42, 387). Unstable in alkaline media.
-
ToxicologyOral Acute oral LD50 for rats >2500 mg/kg.Skin and eye Acute percutaneous LD50 for rats >3000 mg/kg. Non-irritating to skin and eyes.No skin sensitisation (guinea pigs).Inhalation LC50 (4 h) for rats >9.49 g/m3air.Environmental ProfileECOTOXICOLOGY
Birds Acute oral LD50 for California quail >2000 mg/kg.Fish Toxic to fish; LC50 (96 h) for yellow perch 2.36, sheepshead minnow 11,bluegill sunfish 17g/l.Daphnia LC50 (48 h) 3.7g/l.Other aquatic spp. LC50 (96 h) for pink shrimp 1.3g/l.Bees Toxic to bees.LD50 (oral) 0.069 g/bee; (contact) 0.015g/bee.
ENVIRONMENTAL FATE
Animals Metabolism in laying hens was principally by ester hydrolysis and oxidation, followed by conjugation (J. Agric. Food Chem., 1989, 37, 800).Plants Metabolism of 14C-resmethrin on glasshouse-grown tomatoes, glasshouse-grown lettuce and field-grown wheat demonstrated very rapid degradation, resulting in no parent residues 5 days after application.Numerous degradation products were observed in very low amounts.Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide

 0
0 Subscribe
Subscribe