-
Common NameTebufenozide
-
中文通用名虫酰肼
-
IUPACN-tert-butyl-N′-(4-ethylbenzoyl)-3,5-dimethylbenzohydrazide
-
CAS3,5-dimethylbenzoic acid 1-(1,1-dimethylethyl)-2-(4-ethylbenzoyl)hydrazide
-
CAS No.112410-23-8
-
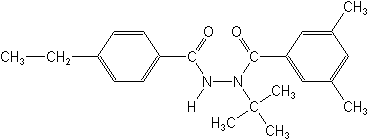
Molecular FormulaC22H28N2O2
-
Molecular Structure
-
Category
-
ActivityInsecticide
Tebufenozide's activity is specific to the larvae of Lepidoptera. It is primarily a stomach poison but it also has some contact activity. Following ingestion, the larvae cease feeding almost immediately. The insecticide mimics the action of the molt hormone, 20-hydroxyecdysone, which causes ecdysis. Artificially raising the concentration of 20-hydroxyecdysone prematurely starts the molting process and the insect dies as a result.
Recommended application times vary according to the species to be controlled: this can be prior to and during egg-laying, or during feeding. Reapplication may be necessary to protect new growth flushes or rapidly expanding fruit. Tebufenozide controls all larval instars but applications to early instars are recommended to prevent the damage that can be inflicted by later instar larvae. In field trials conducted in New York on apple cultivars heavily infested with Choristoneura rosceana, late (fourth and fifth) instars were found to be three times more tolerant to tebufenozide than neonates. Best results are obtained when applications are made at the first signs of feeding damage or when threshold levels of moths, eggs or larvae occur. Tebufenozide also affects reproductive processes.
The product is not plant-systemic and is rainfast once it has dried (6 hours minimum). The addition of the spreader-sticker, Latron may improve distribution on the leaf surfaces and rainfastness. It has residual activity lasting 14 days. The company recommends the use of pheromone traps to indicate optimum spray times.
In field trials conducted in British Columbia, the resistance to tebufenozide of neonate F1 progeny of Choristoneurarosaceana from organic and conventionally managed orchards was determined using a leaf disk bioassay. Insects collected from organic sites were found to be more susceptible than insects collected from conventional sites. As the dose-response regression lines for tebufenozide were parallel, this suggests that that the resistance mechanisms are quantitatively but not qualitatively different. Three other insecticides were tested: azinphosmethyl, methoxyfenozide (P0071) and indoxacarb (P0076); cross-resistance between tebufenozide and azinphosmethyl was found across populations. In other field trials, tebufenozide did not damage fruit crops when applied at the recommended rates.
Tebufenozide is safe to pollinators and predatory insects and mites and the product can be used to control insects resistant to applications of OP's, carbamates or pyrethroids. The compound is recommended for use in IPM schemes, and is a Group 16A insecticide. -
CropUseCropUses:
apples, avocados, blueberries, brassicas, bush berries, cane berries, citrus, citrus oil, cole crops, cotton, cranberries, custard apples, eucalyptus, forestry, fruits, fruiting vegetables, garden beets, gooseberries, huckleberries, kiwifruit, leafy vegetables, legumes, mecadamia nuts, mint, noncropped areas, ornamentals, pecans, pome fruits, rice, shrubs, sugarcane, sunflowers, table beets, tree nuts, trees, turnips, walnuts, vines
Vines
144 g ai/ha
Forestry
70-140 g ai/ha
:Apples
14.4-350 g ai/hl
Walnuts
280 g ai/ha
Vegetables
67-135 g ai/ha
-
PremixTebufenozide+Indoxacarb
Tebufenozide+beta-cypermethrinType
AI concn
Suspension concentrate (SC)
20% (w/v)
24% (w/v)
Wettable powder (WP)
70% (w/w)
Driftless dust (DL)
0.75% (w/w)
Low volume (LV)
20% (w/v)
-
Physical PropertiesMolecular weight:352.5; Physical form:Off-white powder. Density:1.03 (20 °C, pycrometer method); Melting point:191 °C; Vapour pressure:<1.56 × 10-4 mPa (25 °C, gas saturation method); Henry constant:<6.59 ×10-5 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 4.25 ( pH 7); Solubility:In water 0.83 ppm (25 °C). Slightly soluble in organic solvents.; Stability:Stable at 94 °C for 7 d. Stable to light in pH 7 aqueous solution (25 °C). Stable in dark, sterile water 30 d (25 °C). DT50 in natural pond water 67 d, in light, 30 d (25 °C).;
-
ToxicologyOral:Acute oral LD50 for rats and mice >5000 mg/kg. Percutaneous:Acute percutaneous LD50 for rats >5000 mg/kg. Non-irritating to eyes and skin (rabbits). Not a skin sensitiser (guinea pigs). Inhalation: LC50 (4 h) for male rats >4.3, female rats >4.5 mg/l. ADI:( JMPR) 0.03 mg/kg b.w. [1994].
-
Environmental ProfileEcotoxicology:
Algae:EC50 (120 h) for Selenastrum >0.64 mg/l; (96 h) for Scenedesmus 0.23 mg/l.Bees:LD50 (96 h, contact) for honeybees >234 µg/bee.Birds:Acute oralLD50 for quail >2150 mg/kg. Dietary LC50 (8 d) for mallard ducks and quail >5000 mg/kg.Daphnia: LC50 (48 h) 3.8 mg/l.Fish: LC50 (96 h) for rainbow trout 5.7, bluegill sunfish 3.0 mg/l.Worms: LC50 for earthworms >1000 mg/kg.Other aquatic spp.: LC50 (96 h) for mysid shrimp (Mysidopsis bahia) 1.4, Eastern oyster (Crassostrea virginica) 0.64 mg/l.Other beneficial spp.:Safe to predatory mites, wasps and other beneficial species.
Environmental fate:
Animals:In the rat, 16 whole-molecule metabolites are formed as a result of oxidation of the alkyl substituents of the aromatic rings, primarily at the benzylic positions.Soil:Metabolic DT50 in soil 7-66 d (7 soil types); for aerobic, aquatic soil 100 d (25 °C, 3 soil types); for anaerobic, aquatic metabolism 179 d (25 °C, silt loam). DT50 for field dissipation 4-53 d (12 siPlant:In apples, grapes, rice and sugar beet, the major component is unchanged tebufenozide. Metabolites which are detected in small amounts result from oxidation of the alkyl substituents of the aromatic ring, primarily at the benzylic positions.
Bobwhite quail
LD50 >2,150 mg/kg
Rainbow trout [96 h]
LC50 5.7 mg/L
Bluegill sunfish [96 h]
LC50 3 mg/L
Bee [contact]
LD50 >234 μg/bee
Earthworm
LD50 >1,000 mg/kg soil
Fate in :
Tebufenozide poses a low hazard to bees and can be applied to plants at any time, but it may affect aquatic organisms.Fate in soil:
Tebufenozide is immobile in soil. Its half-life is between 31 and 52 days.Fate in aquatic systems:
Tebufenozide's half-life in pond water is less than three days.
The company states that as no acute dietary endpoint was determined, harm from acute exposure from drinking water is unlikely. The Estimated Environmental Concentrations (EECs) were calculated using computer modelling (GENEEC and SCI-GROW programmes) and found to be 16.5 ppb for surface water and 1.04 ppb for ground water for the chronic scenario. These values were less than the lowest drinking water level of concern (DWLOC) value of 49 ppb. -
Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)
Porduct NewsMore
Canada issued re-evaluation plan for insecticide tebufenozide
Related CompaniesMore
Shandong Jingbo Agrochemicals Technology Co., Ltd.
Country: China
Pyrimethanil Pyraclostrobin Fomesafen Tebufenozide Fenoxanil Azoxystrobin Kresoxim-methyl Boscalid Nicosulfuron Emamectin benzoate Indoxacarb Quizalofop-P-ethyl
Jiangsu Baoling Chemical Co., Ltd.
Country: China
Metalaxyl-M Propamocarb Tebufenozide Metalaxyl Chlorpyrifos Phoxim Profenofos Tricyclopyricarb
Shandong Zhongxin Kenong Biological Technology Co., Ltd
Country: China
Lambda-cyhalothrin Imidacloprid Acetamiprid Emamectin benzoate Abamectin Pymetrozine Propiconazole Iprodione Carbendazim+mancozeb Azoxystrobin+difenoconazole Diquat
Country: China
Fipronil Spirodiclofen Florasulam Desmedipham Phenmedipham Ethofumesate Glufosinate-ammonium Diquat dibromide Paraquat dichloride Pyraclostrobin
Xi’an Hytech Agrochemicals Co.,Ltd.
Country: China
Benziothiazolinone Benziothiazolinone Oligosaccharins Thiamethoxam 11.6%+Pymetrozine28.4% Thiamethoxam 11.1% + Lambda-cyhalothrin 14.9% Clothianidin 0.6% + Cyfluthrin 0.4% Pyraclostrobin 5.9% + Propineb 53.1% Tebufenozide Bifenthrin Pyraclostrobin

 0
0 Subscribe
Subscribe