-
Common NameTralomethrin
-
中文通用名四溴菊酯
-
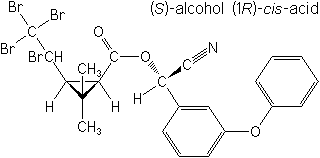
IUPAC(S)-α-cyano-3-phenoxybenzyl (1R,3S)-2,2-dimethyl-3-[(RS)-1,2,2,2-tetrabromoethyl]cyclopropanecarboxylateor(S)-α-cyano-3-phenoxybenzyl (1R)-cis-2,2-dimethyl-3-[(RS)-1,2,2,2-tetrabromoethyl]cyclopropanecarboxylate
-
CAS(S)-cyano(3-phenoxyphenyl)methyl (1R,3S)-2,2-dimethyl-3-(1,2,2,2-tetrabromoethyl)cyclopropanecarboxylate
-
CAS No.66841-25-6
-
Molecular FormulaC22H19Br4NO3
-
Molecular Structure
-
Category
-
Activity
-
CropUseTralomethrin was a broad-spectrum Type II systemic pyrethroid ester insecticide that was registered for use in a variety of residential and commercial settings, and on a small number of agricultural crops including broccoli, cauliflower, cotton, lettuce, peanuts, and sunflowers.
-
Disease Spectrum
-
Weed Spectrum
-
Pest Spectrum
-
FormulationSC = Suspension concentrate (=flowable concentrate)
WP = Wettable powder -
Premix
-
Physical PropertiesMolecular weight:665.0; Physical form:Yellow to beige, resinous solid ( tech.). Density:1.70 (20 °C); Composition:Laboratory grade tralomethrin (>93% a.i.) is a 60:40 mixture of two active diastereoisomers. Melting point:138-148 °C; Flash point:26 °C; Vapour pressure:4.8 × 10-6 mPa (25 °C); Partition coefficient(n-octanol and water):logP = c. 5 (25 °C); Solubility:In water 80 µg/l. In acetone, dichloromethane, toluene, xylene >1000, dimethyl sulfoxide >500, ethanol >180 (all in g/l).; Stability:Stable for 6 months at 50 °C. Acidic media reduce hydrolysis and epimerisation.;
-
ToxicologyOral:Acute oral LD50 for rats 99-3000 mg a.i./kg, depending on the carrier used; for dogs >500 mg (in capsules)/kg. Percutaneous:Acute percutaneous LD50 for rabbits >2000 mg/kg. Moderate skin irritant; mild eye irritant (rabbits). Inhalation:LC50 (4 h) for rats >0.40 mg/l air.
-
Environmental ProfileEcotoxicology:
Bees:Non-toxic to honeybees. LD50 (contact) 0.12 µg/bee. Low LC50 and LD50 values under laboratory conditions do not represent significant hazard to bees in normal fBirds:Acute oral LD50 for quail >2510 mg/kg. Dietary LC50 (8 d) for mallard ducks 7716, quail 4300 mg/kg diet.Daphnia: LC50 (48 h) 38 ng/l. Low LC50 and LD50 values under laboratory conditions do not represent significant hazard to aquatic fauna in normal field use.Fish: LC50 (96 h) for rainbow trout 0.0016, bluegill sunfish 0.0043 mg/l.
Environmental fate:
Animals:Tralomethrin is transformed into deltamethrin and further degraded.Soil:Strongly adsorbed in soil; DT50 64-84 d.Kd 197-8784; Koc 43 796-675 667; highly immobile in various soils (sandy to clay loam).Plant:Tralomethrin is transformed into deltamethrin. -
Transport InformationHazard Class:II(Moderately hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide
Related CompaniesMore
Country: China
Clethodim Hexazinone Mesotrione Abamectin Acetamiprid Dimethoate Fipronil Imidacloprid Fosetyl-aluminium 2,4-D

 0
0 Subscribe
Subscribe