-
Common NameBioallethrin
-
中文通用名Es-生物烯丙菊酯
-
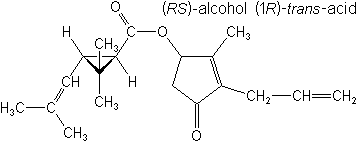
IUPAC(RS)-3-allyl-2-methyl-4-oxocyclopent-2-enyl (1R,3R)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
or
(RS)-3-allyl-2-methyl-4-oxocyclopent-2-enyl (1R)-trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate -
CAS2-methyl-4-oxo-3-(2-propenyl)-2-cyclopenten-1-yl (1R,3R)-2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylate
-
CAS No.584-79-2
-
Molecular FormulaC19H26O3
-
Molecular Structure
-
Category
-
ActivityInsecticide
Bioallethrin is a pyrethroid chemical used as an insecticide. The chemical is toxic to the nerve system. Non-systemic, contact and stomach action witha rapid knock-down effect. Sodium channel modulator. -
FormulationAE = Aerosol dispenser
-
PremixPermethrin+bioallethrin+Piperonyl butoxide
Permethrin+bioallethrin
Deltamethrin+s-bioallethrin+Piperonyl butoxide
Deltamethrin+s-bioallethrin
Deltamethrin+bioallethrin
Bioallethrin+zeta-cypermethrin
Bioallethrin+Piperonyl butoxide+tetramethrin
Bifenthrin+s-bioallethrin
-
Physical PropertiesMolecular weight:302.4; Physical form:Bioallethrin is an orange-yellow viscous liquid. The material in 'D-Trans' is an amber viscous liquid. Density:1.012 at 20 °C; Composition:Bioallethrin contains ×93% m/m total isomers, of which there is ×90% trans- isomer pair and ×3% cis- isomer pair. Material in 'D-Trans' is 90% m/m bioallet Melting point:Not applicable; no crystallisation observed at -40 °C; Flash point:87 °C (Pensky-Martens); Vapour pressure:43.9 mPa (25 °C); Henry constant:2.89 Pa m3 mol-1 (calc.); Partition coefficient(n-octanol and water):logP = 4.68 (25 °C); Solubility:Bioallethrin: in water 4.6 mg/l (25 °C). Completely miscible with acetone, ethanol, chloroform, ethyl acetate, hexane, toluene and dichloromethane (20 °C).; Stability:Degraded by u.v. light. In aqueous solution, DT50 1410.7 d (pH 5), 547.3 d ( pH 7), 4.3 d ( pH 9).;
-
ToxicologyOral:Acute oral LD50 for male rats 709 mg bioallethrin/kg, female rats 1042 mg/kg; for male rats 425-575 mg< ('D-Trans')/kg, female Percutaneous:Acute percutaneous LD50 for rabbits >3000 mg bioallethrin/kg. Inhalation: LC50 (4 h) for rats 2.5 mg bioallethrin/l air. (Rat): Oral LD50 860 mg/kg
-
Environmental ProfileEcotoxicology:
Birds:Acute oral LD50 for bobwhite quail 2030 mg/kg.Daphnia:LC50 (96 h) 0.0356 mg/l.Fish:Highly toxic. LC50 (96 h) (static test, flow-through test) for coho salmon 22.2, 9.40, steelhead trout 17.5, 9.70, channel catfish >30.1, 27.0, yellow perch -, 9.90mg/l.
Environmental fate:
Animals:[14C-acid]-bioallethrin administered in rats with a single dose of either 200 or 100 mg/ kg b.w. was readily eliminated in the urine and faeces of all dose groups within 2-3 days after treatment.Soil:[14C-alcohol]-bioallethrin degrades in pH 5 buffer to allethrolene, dihydroxyallethrolene, CO2 and a number of small yield polar products as the result of photochemical processes. Cis/trans isomerisation was -
Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide
Related CompaniesMore
Country: China
Brodifacoum Bromadiolone Difenacoum Chloralose Transfluthrin Tetramethrin Meperfluthrin Bioallethrin Dimefluthrin Prallethrin

 0
0 Subscribe
Subscribe