-
Common NameClothianidin
-
中文通用名噻虫胺
-
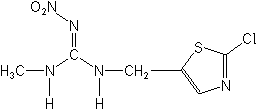
IUPAC(E)-1-(2-chloro-1,3-thiazol-5-ylmethyl)-3-methyl-2-nitroguanidine
-
CAS[C(E)]-N-[(2-chloro-5-thiazolyl)methyl]-N′-methyl-N″-nitroguanidine
-
CAS No.210880-92-5 (formerly 205510-53-8)
-
Molecular FormulaC6H8ClN5O2S
-
Molecular Structure
-
Category
-
ActivityInsecticide.
Clothianidin is a broad spectrum, highly systemic insecticide, suitable for application to soil, foliage and seeds. Plant uptake occurs via the cotyledons and roots of emerging seedlings and through the roots of established plants. Translocation studies (BCPC, 2002) showed that clothianidin moves in an acropetal and basipetal manner. Material taken up by the roots is transported rapidly to the leaves. Translaminar activity results in efficient transport of the product across leaf tissues from one surface to the other.
Ingestion of plant material by pests results in rapid cessation of feeding then death. The product is effective against pests from the orders Hemiptera, Coleoptera, Thysanoptera, Diptera and some Lepidoptera. In biochemical mode of action studies, clothianidin was found to be a potent agonist of the Drosophila SAD / chicken β2 hybrid nicotinic acetylcholine receptor. No activation was observed on the chicken neuronal α4β2 nicotinic acetylcholine receptor (BCPC, 2002).
In field trials conducted in the US cornbelt between 1997 and 2000, clothianidin was shown to provide control of Western, Northern, Southern and Mexican corn rootworm (Diabrotica spp) at a level comparable to existing commercial organophosphorus (e.g. chlorpyrifos) and pyrethroid (e.g. tefluthrin) products. Tomen's US regulatory submission refers to control of aphids, apple maggots, leafminers, leafrollers, leafhoppers, codling moths and pear psylla.
Clothianidin exhibits good application flexibility. Foliar applications of water soluble granules at 50-100 mg ai/L were shown to control aphids on eggplants for more than 3 weeks while application of granular formulations at 50-100 g ai/ha to paddy water resulted in excellent residual control of brown rice planthoppers (Nilaparvata lugens) and green rice leafhoppers (Nephotettix cincticeps). Rice seedlings treated just before transplantation into seedling boxes with clothianidin at 150 g ai/ha were protected from damage by brown planthoppers. Granular formulations can also be sprinkled at 56 mg ai/plant into planting holes prior to, or just after planting to protect vegetables such as eggplant against attack by aphids. In greenhouse cultivation, the product can be used in various application methods at 2.5 mg ai/plant to control whitefly for over 56 days on tomatoes.
The seed treatment, Poncho 250 is applied at the low rate of 0.25 mg ai / kernel in maize and provides early season seed and seedling protection against cutworms (Agrotis spp), wireworms (Melanotus spp), seed corn maggots (Hylemyia platura), white grubs (Phyllophaga spp), chinch bugs (Blissus leucopterus), flea beetles (Chaetocnema pulicaria) and numerous other pests. It is reported to produce average yields of 8.4 bushels / acre more than untreated seed and 4 bushels / acre more than seed treated with Gaucho (imidacloprid). Poncho 1250, applied at 1.25 mg ai / kernel, also protects against corn rootworm (Diabrotica spp) and extends the period of protection for seedlings. In field trials, use of Poncho 1250 resulted in average production yields of 12.6 bushels / acre more than untreated seed. The control of corn rootworm is reported to be superior to that given by Prescribe (imidacloprid). In canola, this seed treatment is effective against flea beetles at rates of 1.5 - 4 g ai / kg seed, depending on pest pressure.
In corn, clothianidin can be applied as a seed treatment at 200-400 g ai/100 kg seed to control wheat aphids (Rhopalosiphum padi). A 600 FS formulation was tested from 1997 to 2001 in the US corn belt and was shown to give consistent control of the corn rootworm complex (Diabrotica spp) with efficacy equal to the commercial standards, fipronil (P0038L) and chlorpyrifos (P0031). Secondary pests also controlled included: wireworm (Melanotus spp); flea beetles (Chaetocnema pulicaria); white grubs (Lachnosterna implicita); grape colaspis (Colaspis brunnea); black cutworm (Agrotis ipsilon); seed corn maggot (Hylemyia platura); corn leaf aphid (Rhopalosiphum maidis); chinch bug (Blissus leocopterus); stink bug (Nezara viridula); and fire ant (Solenopsis spp).
Clothianidin has been extensively field tested in the UK and Europe for its potential as a seed treatment for pest control in sugar beet (BPCP, 2002). Comparisons with the industry reference, imidacloprid, showed that clothianidin provided good control of green aphid (Myzus persicae) for up to 10 weeks after sowing and reduced the incidence of yellowing virus attacks. The efficacy of clothianidin was comparable to imidacloprid. Further studies provided no evidence of cross-resistance between clothianidin and known resistance mechanisms. Mixtures with beta-cyfluthrin will offer lower rates, faster action and broader spectrum than imidacloprid.
Clothianidin has been extensively tested in development field trials in France. It has shown excellent activity against woolly apple aphid (Eriosoma lanigerum) and rosy apple aphid (Dysaphis plantaginea) on apples and pears, green peach aphid(Myzus persicae)on peachesand the aphid complex (Macrosiphum euphorbiae, Aphis nasturtii, Aulacorthum solanii, Myzus persicae, Aphis frangulae) on potatoes. Treatments made with 75 g ai/ha provide wide spectrum aphid control including certain biotypes resistant to existing commercial products and good residual activity. -
CropUseCrop uses:
aubergines, apples, citrus, corn, cotton, cucumber, grape, Japanese eggplants, lettuce, maize, melons, canola oilseed rape, ornamentals, peaches, pears, potatoes, raisins rapeseed, raw agricultural commodities, rice, sugar beet, tea, tobacco, tomatoes, turf, watermelons
Rice
Dust formulations applied at 30-40 kg / ha Granule (1 kg) applied at 10 kg / ha 1.0 g / plant (ornamentals) 60 kg / plant (potatoes) 1-2 g / plant (cucumbers, melons, tomatoes, aubergines)
-
Pest SpectrumEarly season pests, soil and leaf pests like aphids, beet leaf miners, black cutworms, corn rootworms, flea beetles, grubs, leafhoppers and wireworms.
-
FormulationFS = Flowable concentrate for seed treatment
GR = Granule
SC = Suspension concentrate (=flowable concentrate)
WG = Water dispersible granules
WP = Wettable powder -
PremixPyridaben+Clothianidin
Pyraclostrobin+Fludioxonil+Clothianidin
Pymetrozine+Clothianidin
Monosultap+Clothianidin
Metalaxyl-M+Prochloraz+Clothianidin
Isoprocarb+Clothianidin
Hexaflumuron+Clothianidin
Fludioxonil+Clothianidin
ethaboxam+clothianidin
Cyromazine+Clothianidin
Clothianidin+Spirotetramat
Clothianidin+metalaxyl+prothioconazole+tebuconazole
Clothianidin+Lambda-Cyhalothrin
Clothianidin+ipconazole+metalaxyl
Clothianidin+Cyfluthrin
Clothianidin+carboxin+metalaxyl+trifloxystrobin
Clothianidin+bifenthrin
Clothianidin+beta-cyfluthrin
Chlorfenapyr+Clothianidin
Chlorantraniliprole+Clothianidin
Abamectin+Clothianidin
-
Physical PropertiesMolecular weight:249.7.
-
Toxicology
Acute oral (rat)
LD50 >5,000 mg/kg. ADI:0.1 mg/kg.
-
Environmental ProfileWATER SOLUBILITY: 0.327 g/l at 20° C.
Bobwhite quail [oral]
LD50 >2000 mg/kg
Bluegill sunfish [96 h]
LC50 >120 mg/L
Bobwhite quail [dietary]
LC50 >5200 ppm
Mallard duck [dietary]
LC50 >5200 ppm
Rainbow trout [96 h]
LC50 >100 mg/L
Daphnia magna[48 h]
EC50 >120 mg/L
Green alga [72 h]
EbC50 >270 mg/L
Earthworms [14 d]
LC50 13.21 mg/kg dry soil
Fate in aquatic systems:
Hydrolysis at 20°C; clothianidin is stable in water at pH 4-7 and shows very slight degradation at pH 9.
Aqueous photolysis; photolytic degradation in sterile buffer was very fast; clothianidin was completely metabolised with a DT50 of < 4 hours. In river water, the photolytic half life was 26.6 days.Fate in soil:
The proposed metabolic pathway for clothianidin in soils involves several degradation products, of which only N-methyl-N'-nitroguanidine (MNG) is a major metabolite. Loss of the guanidine methyl group produces N-(2-chlorothiazol-5-ylmethyl)-N'-nitroguanidine (TZNG), while cleavage of the nitroguanidine group produces MNG. Demethylation of MNG and cleavage of TZNG leads to nitroguanidine (NTG). Loss of the nitroimino group from clothianidin produces N-(2-chlorothiazol-5-ylmethyl)-N'-methylurea (TZMU).
In aerobic laboratory soil metabolism studies on 9 soils, the DT50 values ranged between 143 and 1328 days with an average value of 518 days . The relevant soil metabolites MNG and TZNG were degraded on 3 soils with half lives of 82-108 days and 62-111 days respectively.
Anaerobic soil studies in a farm pond model system maintained for one year gave a half life for clothianidin of 21 days in the entire system (rapid degradation). Clothianidin is rapidly transferred to the soil layer where it becomes increasingly strongly bound. At the end of the study, 1.4% of the applied radioactivity remained in the water phase while in the sediment, the radioactivity level increased to more than 90% within 59 days. Clothianidin was the only product detected in the water phase.
Laboratory aerobic water-sediment studies in two pond systems (representing a gravel pit and a dammed pond) were conducted for 100 days. Clothianidin was moderately degraded with half-lives of 48 days and 65 days for the entire systems. Most of the radioactivity was transferred to the sediments. Clothianidin levels in the water at the end of the studies amounted to 9% and 19% of the applied radioactivity, while the bound residues on the sediments reached 30% and 43%, identified as clothianidin and the metabolite TMG. No metabolite exceeded 1.3% of applied radioactivity.
Photolysis on a loamy sand soil surface; a significant degradation process producing many metabolites in low quantities; half-life of clothianidin = 8.2 days (equivalent to 34 days of sun in Phoenix, USA).
Adsorption / desorption studies in five soils (organic carbon content = 0.4-2.1%) resulted in clothianidin being classified as low to intermediate mobility with a mean adsorption Koc value of 160 mL/g. The desorption Koc was 188 mL/G. After aging for 99 days on soil, the Koc values increased by a factor of 2.1 to 3.5 indicating increased binding to soil. For the major metabolites MNG and TZNG, the mean Koc values in the same non-aged soils were 21 mL/g and 275 mL/g respectively; MNG is classified as mobile while TZNG is classified of low mobility.
Lysimeter studies conducted over 3 years on a sandy loam (72.5% sand) showed no clothianidin in the leachates. Metabolites were detected but none exceeded the EU trigger level of 0.1 mg/L.
Six field dissipation studies were conducted in N Europe; 4 on bare soil and 2 in cropped situations. Two further studies were performed in S Europe on cropped soil. Clothianidin was applied as a single spray of 150 g ai/ha. A mean DT50 of 120 days and a mean DT90of 399 days was calculated. The N European trials gave a mean DT50 of 75 days. Practically all residues remained in the upper 10 cm soil. Clothianidin was classified as moderately degradable in soil under field conditions. -
Transport InformationHazard Class:O (Obsolete as pesticide, not classified)
Porduct NewsMore
US EPA issues proposed interim decision on neonicotinoids, opens comments
Clothianidin Thiamethoxam Imidacloprid
Valent to offer new insecticide, herbicide in 2020
Clothianidin Glufosinate-ammonium
Canada to phase out three neonicotinoid pesticides over the next two to three years
Clothianidin Imidacloprid Thiamethoxam
UK rejects emergency sugar beet neonics use applications
UK confirmed withdrawal dates for three neonicotinoid insecticides
Imidacloprid Clothianidin Thiamethoxam
Sumitomo Chemical reiterates the effectiveness and safety of clothianidin
Bayer launches trial to fight against malaria in Mozambique
Canada proposes to grant three-year registration for clothianidin and thiamethoxam
Sumitomo Chemical’s SumiShield™ 50WG insecticide receives WHO prequalification
Bayer launches Poncho Energy technology for seed protection in Brazil
Related CompaniesMore
SINO CHEMTECH (SHANGHAI) CO., LTD
Country: China
Tribenuron-methyl Nicosulfuron Dimethomorph Clodinafop-propargyl Diazinon Emamectin benzoate Imidacloprid Thiamethoxam Chlorpyrifos+cypermethrin Bromoxynil octanoate+MCPA-isooctyl BAAPE IR3535
HEBEI BRILLIANT CHEMICAL CO ., LTD.
Country: China
Thiamethoxam Clothianidin Spirodiclofen 2-chloro-5-chloromethylthiazole Oxadiazine
Country: China
MCPA Cyproconazole Imazamox Flumetsulam Haloxyfop-P-methyl Penoxsulam Florasulam Prothioconazole Clothianidin
Hangzhou Ruijiang Crop Science Co., Ltd
Country: China
Glyphosate 2,4-D Paraquat Nicosulfuron Imidacloprid Carbendazim Dicamba+2,4-D Pyraclostrobin Spirodiclofen Seaweed Extract Amino Acid Agriculural Organosilicone Adjuvant
Country: China
Glufosinate-ammonium 2,4-D MCPA Dicamba Propanil Clethodim Glyphosate Captan Flumioxazin Sulfentrazone

 0
0 Subscribe
Subscribe