-
Common NameDeltamethrin
-
中文通用名溴氰菊酯
-
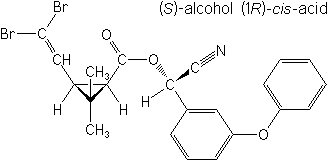
IUPAC(S)-α-cyano-3-phenoxybenzyl (1R,3R)-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropanecarboxylate
or
(S)-α-cyano-3-phenoxybenzyl (1R)-cis-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropanecarboxylate -
CAS(S)-cyano(3-phenoxyphenyl)methyl (1R,3R)-3-(2,2-dibromoethenyl)-2,2-dimethylcyclopropanecarboxylate
-
CAS No.52918-63-5
-
Molecular FormulaC22H19Br2NO3
-
Molecular Structure
-
Category
-
ActivityDeltamethrin is a pyrethroid insecticide that kills insects on contact and through digestion. It is used to control apple and pear suckers, plum fruit moth, caterpillars on brassicas, pea moth, aphids (apples, plums, hops), winter moth (apples and plums), codling and tortrix moths (apples). Control of aphids, mealy bugs, scale insects, and whitefly on glasshouse cucumbers, tomatoes, peppers, potted plants, and ornamentals. It also controls numerous insect pests of field crops.
-
CropUseMore than 150 crops including vegetables, fruits, cereals, oil seed rape, rice, soybeans and corn
-
Pest SpectrumWide range of insect pests (most of Lepidoptera spp. and Homoptera spp., Coleoptera spp., Orthoptera spp. like locust and grass-hoppers, Diptera spp. like flies, Hemiptera spp. like bugs, Thysanoptera spp. like thrips).
-
FormulationGR = Granule
SL = Soluble concentrate
SC = Suspension concentrate (=flowable concentrate)
WG = Water dispersible granules
WP = Wettable powder -
PremixQuinalphos+deltamethrin
Phoxim+deltamethrin
Malathion+deltamethrin
Isocarbophos+deltamethrin
IMIPROTHRIN+DELTAMETHRIN
Imidacloprid+deltamethrin
Fipronil+deltamethrin
Fenitrothion+deltamethrin
Endosulfan+deltamethrin
Dichlorvos+deltamethrin
Deltamethrin+triazophos
Deltamethrin+s-bioallethrin+Piperonyl butoxide
Deltamethrin+s-bioallethrin
Deltamethrin+omethoate
Deltamethrin+Oleum Anisi Stellati
Deltamethrin+fenobucarb
Deltamethrin+dimethoate
Deltamethrin+chlorpyrifos-methyl
Deltamethrin+chlorpyrifos
Deltamethrin+bioallethrin
Abamectin+deltamethrin
-
Physical PropertiesMolecular weight:505.2. Physical form:Colourless and odorless crystals. Density:Bulk density 0.55 g/cm3 (25 °C); Composition:Tech. produced industrially by Aventis contains 98% deltamethrin ( i.e. the single isomer). Melting point:100-102 °C; Vapour pressure:1.24×10-5 mPa (25 °C, gas saturation method); Henry constant:3.13×10-2 Pa m3 mol-1 ( calc.). Partition coefficient(n-octanol and water):logP = 4.6 (25 °C); Solubility:In water <0.2 µg/l (25 °C). In dioxane 900, cyclohexanone 750, dichloromethane 700, acetone 500, benzene 450, dimethyl sulfoxide 450, xylene 250, ethanol 15, isopropanol 6 (all in g/l, 20 °C). Stability:Extremely stable on exposure to air. Stable 190 °C. Under u.v. irradiation and in sunlight, a cis-trans isomerisation, splitting of the ester bond, and loss of bromine occur. Stability very good; no breakdown after 6 months at 40°C. Soluble in acetone, ethanol, dioxan, and most aromatic solvent.
-
ToxicologyOral:Acute oral
LD50 for rats ranges from 135->5000 mg/kg, depending upon carrier and conditions of the study; for dogs >300 mg/kg. Percutaneous:Acute percutaneous LD50 for rats and rabbits >2000 mg/kg. Non-irritating to skin; mild eye irritant (rabbits). Inhalation: LC50 (4 h) for rats 2.2 mg/l air; (1 h) >4.6 mg/l air (micronised). -
Environmental ProfileEcotoxicology:
Algae:EC50 (96 h) for algae (Selenastrum capricornutum) >9.1 mg/l. Low LD50 and LC50 values under laboratory conditions do not represent all. Bees:Toxic to bees, LD50 (oral) 79 ng/bee; (contact) 51 ng/bee. Low LD50 and LC50 values under laboratory conditions do not represent a signifBirds:Acute oral LD50 for mallard ducks >4640 mg/kg. Dietary LC50 (8 d) for mallard ducks >8039, quail >5620 mg/kg diet. NOEL for reproduDaphnia: LC50 (48 h) 3.5 µg/l.Fish:Toxic to fish under laboratory conditions; LC50 (96 h) for rainbow trout 0.91, bluegill sunfish 1.4 µg/l. Not toxic to fish under natural conditions.Worms: LC50 (14 d) for earthworms >1290 mg/kg soil.
Environmental fate:
Animals:In rats, following oral administration, elimination occurs within 2-4 days. The phenyl ring is hydroxylated, the ester bond hydrolysed, and the acid moiety is eliminated as the glucuronide and glycine conjugates.Soil:In soil, undergoes microbial degradation within 1-2 weeks.Kd 3790-30 000, Koc 4.6×105-1.63×107 cm3/g, confirms strong aPlant:No uptake through leaves and roots - non-systemic compound. No major metabolites, except in oily crops, where trans-deltamethrin is part of the residue definition.
WATER SOLUBILITY: Dispersible. -
Transport InformationSignal Word:WARNING; Hazard Class:II (Moderately hazardous)
Porduct NewsMore
Canada approves Bayer’s Annihilator PolyZone insecticide
Canada proposes to approve insecticide deltamethrin
Bayer launches trial to fight against malaria in Mozambique
Bayer provides solutions for protection of stored grain in Brazil
Sipcam released 3 products in China
Emamectin benzoate Flubendiamide Deltamethrin
Related CompaniesMore
Country: India
Permethrin Imidacloprid Cypermethrin Chlorpyrifos Alpha-cypermethrin Triclopyr Deltamethrin Fluroxypyr Quinalphos Temephos
Tagros Chemicals India Pvt Ltd
Country: India
Permethrin Deltamethrin ALPHACYPERMETHRIN Cypermethrin Metamitron Transfluthrin Tefluthrin Dicamba Sulfentrazone Fipronil Propiconazole
Ningxia Lamtin Agricultural Development Co.Ltd.
Country: China
Oxadiargyl Bentazone Oxadiazon Metamifop Propanil
Country: India
Lime Sulfur Solution/Calcium Polysulfide Solution Emamectin benzoate Azadirachtin Hexaconazole Mancozeb Pendimethalin Profenophos Thiamethoxam Deltamethrin Gibberellic acid
Country: India
Cypermethrin Permethrin Profenofos 2,4-D Acid Bifenthrin Triclopyr Propanil Hexaconazole Sulphur Thiophanate-methyl

 0
0 Subscribe
Subscribe