-
Common NameDinotefuran
-
中文通用名呋虫胺
-
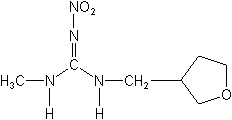
IUPAC(EZ)-(RS)-1-methyl-2-nitro-3-(tetrahydro-3-furylmethyl)guanidine
-
CASN-methyl-N′-nitro-N″-[(tetrahydro-3-furanyl)methyl]guanidine
-
CAS No.165252-70-0
-
Molecular Structure
-
Category
-
ActivityInsecticide.
Dinotefuran can be applied to foliage or to the soil and is active by contact and ingestion. It also has root-systemic activity. The products activity persists for up to several weeks after application.
Dinotefuran has demonstrated no cross-resistance to organophosphates, carbamates, pyrethroids and insect growth regulators. Mitsui recommends its use in both resistance management and integrated pest management systems. In tests against several strains of mosquito species, although it was generally less toxic than standards, dinotefuran was more effective against a carbamate-resistant strain of Culex quinquefasciatus than the susceptible strain (J Med Entomology, 2004).
Data from Mitsui indicate that dinotefuran provides superior control of stinkbugs, fruit moths, flea beetles and leaf miners compared with imidacloprid.
Field trials conducted on golf courses in Mississippi in 2000 with dinotefuran (code numbered YW-74G) demonstrated a comparable level of control of mole crickets (Scapteriscus spp) to the reference product, orthene. -
CropUseCrop uses:
apples, aubergines, brassicas, cabbages, celery, cole crops, cotton, cucumbers, curcubits, field crops, fruiting vegetables, fruit trees, grapevines, leafy vegetables, meat, milk ornamentals, parsley, peppers, potatoes, raisins, rice, sugar beet, swiss chard, tomatoes, turf, vegetable crops, vines, watermelons
100-200 g ai/ha
150-600 g ai/ha by soil application
112-224 g/380 L (Safari, foliar application)
680 g/380 L (Safari; soil drench)
-
FormulationGR = Granule
PB = Plate bait
SC = Suspension concentrate (=flowable concentrate) -
PremixThifluzamide+Dinotefuran
Spirotetramat+Dinotefuran
Pyriproxyfen+Dinotefuran
Pymetrozine+Dinotefuran
Nitenpyram+Dinotefuran
Lambda-cyhalothrin+Dinotefuran
Dinotefuran+Thiamethoxam
Dinotefuran+Pyridaben
Dinotefuran+Isoprocarb
Dinotefuran+Chlorpyrifos
Buprofezin+Dinotefuran
Bifenthrin+Dinotefuran
Abamectin+Dinotefuran
-
Physical PropertiesMolecular weight:202.2.Physical form: White solid; odorless. Density:1.33; Melting point:94.5-101.5 °C; Partition coefficient(n-octanol and water):logP = -0.644 ( pH 7); pKa:No dissociation in range pH 1.4 to 12.3; Solubility:In purified water 54.3 .3 g/l (20 °C).
-
ToxicologyOral:Acute oral
LD50 for male rats 2804 mg/kg, female rats 2000 mg/kg, male mice 2450 mg/kg, female mice 2275 mg/kg. Percutaneous:Acute percutaneous LD50 for male and female rats >2000 mg/kg. Not a skin sensitiser (guinea pigs). ADI:( JMPR) 0.008 mg/kg b.w. [2000].?
-
Environmental ProfileEcotoxicology:?
Birds:Acute oralLD50 for mallard ducks 1000, Japanese quail >2000 mg/kg.Daphnia:(48 h) 1000 ppm.Fish: LC50 (96 h) for carp >1000 ppm; (48 h) for rainbow trout >40 ppm.Other aquatic spp.: LC50 (48 h) for crayfish 5-10 ppm.
WATER SOLUBILITY: 39.8 g/l at 20°C.
Mallard duck
LD50 1,000 mg/kg
Japanese quail
LD50 >2,000 mg/kg
Rainbow trout [48 h]
LC50 >100 mg/L
Rainbow troutchronic
NOEC >/= 10 mg/L
Bluegill sunfish acute
LC50 >100 mg/L
Carp [96 h]
LC50 >100 mg/L
Daphnia magna acute
EC50 >1000 mg/L
Daphnia magna chronic
EC50 >100 mg/L
Sheeps head minnow acute
LC50 >109 mg/L
Mysid shrimp acute
LC50 = 0.79 mg/L
Oyster acute
EC50 >141 mg/L
Fate in aquatic systems:
Hydrolysis at 20C; dinotefuran is stable in water at pH 4, 7 and 9.
Aqueous photolysis; photolytic degradation in sterile buffer gave a DT50 of 1.7 days at Summer latitude 30N and 18.60 days at winter latitude 50N.Fate in soil:
In aerobic laboratory soil metabolism studies on loamy sand soil, the DT50 value was 51.7 days.
In a 320 day aquatic water-sediment system, the DT50 value for dinotefuran in the aerobic water phase was 23-49 days and 45-128 days in the largely anaerobic sediment layer.
Field dissipation studies in sandy loam soils in the US gave DT50 values of 68.6 days in California (pH 8.8), 22.6 days in Georgia (pH 6.5) and 59.2 days (pH 6.6). -
Transport InformationHazard Class:O (Obsolete as pesticide, not classified)
Porduct NewsMore
Brazil: Ihara to launch new insecticide Zeus at Cocari Field Day
Ihara announces registration of new molecule in Brazil
US court rejects California’s approval of dinotefuran
US EPA received specific exemption requests for dinotefuran
Summit Agro launches Tenchu insecticide in Argentina
Australia to approve AgNova’s insecticide dinotefuran
Related CompaniesMore
SINO CHEMTECH (SHANGHAI) CO., LTD
Country: China
Tribenuron-methyl Nicosulfuron Dimethomorph Clodinafop-propargyl Diazinon Emamectin benzoate Imidacloprid Thiamethoxam Chlorpyrifos+cypermethrin Bromoxynil octanoate+MCPA-isooctyl BAAPE IR3535
Yancheng Limin Chemical Co.,Ltd
Country: China
Triadimefon Triadimenol Hexaconazole Flutriafol Imidacloprid Paclobutrazol Tebuconazole Thifluzamide Indoxacarb Uniconazole
Jiangsu Subin Agrochemical Co., Ltd.
Country: China
Thiamethoxam Pymetrozine Fludioxonil Dinotefuran Lufenuron+Emamectin benzoate Emamectin benzoate Azoxystrobin+cyproconazole Teflubenzuron Thiamethoxam+lambda-cyhalothrin
Shanghai Heben-Eastsun Medicaments Co., Ltd.
Country: China
Thiamethoxam Azoxystrobin Boscalid Chlorfenapyr Difenoconazole Fludioxonil Indoxacarb Picoxystrobin Pyraclostrobin Tebuconazole
Shandong Kangqiao Bio-technology Co., Ltd.
Country: China
Pyraclostrobin+boscalid Pyraclostrobin+metiram Pyraclostrobin+epoxiconazole Thifluzamide Spiromesifen Spirodiclofen Pyraclostrobin

 0
0 Subscribe
Subscribe