-
Common NamePropoxur
-
中文通用名残杀威
-
IUPAC2-isopropoxyphenyl methylcarbamate
-
CAS2-(1-methylethoxy)phenyl methylcarbamate
-
CAS No.114-26-1
-
Molecular FormulaC11H15NO3
-
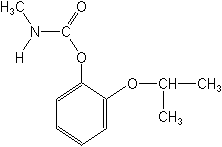
Molecular Structure
-
Category
-
ActivityInsecticide
-
PremixPropoxur+Lambda-cyhalothrin
Aerosol, bait, emulsifiable concentrate, dustable powder, fumigant, granules, oilspray, ULV liquid, wettable powder. Hercon* Toxstrip BW in controlled release strip formulation; insecticide is present on the active surface and in the inner reservoir of the multi-layered, controlled release, laminated polymeric strips. Premix Parters: ioxynil;
-
Physical PropertiesMolecular weight:209.2; Physical form:Colourless crystals; ( tech., white to cream-coloured crystals). Density:1.18 (20 °C); Melting point:90 °C (crystal form I), 87.5 °C (crystal form II, unstable); Vapour pressure:1.3 mPa (20 °C); 2.8 mPa (25 °C); Henry constant:1.5 × 10-4 Pa m3 mol-1 (20 °C); Partition coefficient(n-octanol and water):logP = 1.56; Solubility:In water 1.9 g/l (20 °C). Soluble in most organic solvents, e.g. isopropanol >200, toluene 50-100, hexane 1-2 (all in g/l, 20 °C).; Stability:Stable in water at pH 7. Hydrolysed by strong alkali; DT50 (22 °C) 1 y ( pH 4), 93 d ( pH 7), 30 h ( pH 9). Compatible with most insecticides, fungicides except alkalines. Unstable in highly alkaline media. Readily soluble in dichloromethane, 2-propanol, toluene. Hardly soluble in n-hexane.
-
ToxicologyOral:Acute oral LD50 for male and female rats c. 50 mg/ kg. Percutaneous:Acute percutaneous LD50 (24 h) for male and female rats >5000 mg/kg. Non-irritating to skin; slightly irritating to eyes (rabbits). Inhalation: LC50 for rats (4 h) >0.5 mg/l (aerosol); 0.654 mg/l air (dust). Phytotoxicity:Chrysanthemums, carnations, and hydrangeas may be injured at higher dose rates. Blossom thinning may occur on fruit trees. ADI:2.5 mg/ kg.
-
Environmental ProfileEcotoxicology:
Bees:Highly toxic to bees.Birds:Dietary LC50 (5 d) for bobwhite quail 2828, mallard ducks >5000 mg/ kg diet.Daphnia: LC50 (48 h) 0.15 mg/l.Fish: LC50 (96 h) for bluegill sunfish 6.2-6.6, rainbow trout 3.7-13.6, golden orfe 12.4 mg/l.
Environmental fate:
Animals:In rats, the principal metabolites are 2-hydroxyphenyl-N-methylcarbamate and 2-isopropoxyphenol; minor metabolites include 5-hydroxy propoxur and N-hydroxymethyl propoxur. Elimination is very rapid, 96% excreted in the urine. Metabolism Soil:The mobility of propoxur in the soil is relatively high. The compound rapidly degrades in different soils.Plant:The primary metabolite is demethyl propoxur (max. amount 2.7-3.6%). WATER SOLUBILITY: 1.75 g/l at 20° C -
Transport InformationSignal Word:WARNING; Hazard Class:II(Moderately hazardous)
Porduct NewsMore
US EPA proposes to cancel some uses of insecticide propoxur
Related CompaniesMore
Country: China
Copper hydroxide Brassinolide Azoxystrobin Metaldehyde Prohexadione-calcium Adjuvant: Super-spreader Organosilicone PASS Adjuvant: Penetrant-Spreader Pro-MSO Plus Super Absorbent Polymer for Agriculture Pro-SAP Adjuvant: Water Conditioner Pro-Buffer L2
Hunan Haili Chemical Industry Co., Ltd.
Country: China
Carbofuran Carbosulfan Propoxur Fenobucarb/BPMC lsoprocarb/MIPC Carbaryl Methomyl Dimethoate Pirimiphos-methyl Thiodicarb Thiophanate-methyl

 0
0 Subscribe
Subscribe