-
Common NameFenbuconazole
-
中文通用名腈苯唑
-
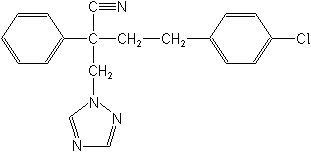
IUPAC(RS)-4-(4-chlorophenyl)-2-phenyl-2-(1H-1,2,4-triazol-1-ylmethyl)butyronitrile
-
CASα-[2-(4-chlorophenyl)ethyl]-α-phenyl-1H-1,2,4-triazole-1-propanenitrile
-
CAS No.114369-43-6
-
Molecular FormulaC19H17ClN4
-
Molecular Structure
-
Category
-
ActivityFungicide
Fenbuconazole is effective against ascomycetes, basidiomycetes and deuteromycetes. Following foliar application, the compound is translocated acropetally. Fenbuconazole has residual activity lasting 4-5 weeks. The optimum timing for protectant applications in wheat is at GS 38-45.
In field trials, fenbuconazole gave similar levels of disease control at lower application rates when compared with other azoles. -
CropUseCropUses:
apples, cereals, fruits (including vines), nuts, oilseed rape, ornamentals, pears, sugar beets, turf, vegetables, vines
Cereals
75-125 g ai/ha
Oilseed rape
60-75 g ai/ha
Sugar beet
65-280 g ai/ha
Peanuts
75-150 g ai/ha
Rice
50-150 g ai/ha
Turf
75-250 g ai/1,000m2
Vines
30-45 g ai/ha
Nuts
18-25 g ai/ha
Fruit
50-75 g ai/ha
Ornamentals
5-10 g ai/ha
Vegetables
50-100 g ai/ha
-
Premix
Emulsifiable concentrate (EC) ;Emulsion-in-water (EW) ;Suspension concentrate (SC);Wettable powder (WP)
Premix Parters:
cypermethrin; dimethoate; esfenvalerate; fenobucarb; fenvalerate; methabenzthiazuron; permethrin; tetramethrin; trichlorfon;
-
Physical PropertiesMolecular weight:336.8; Physical form:Colourless crystals. Melting point:124-126 °C; Vapour pressure:0.005 mPa (20 °C); Partition coefficient(n-octanol and water):logP = 3.23; Solubility:In water 0.2 mg/l (25 °C). Soluble in common organic solvents such as ketones, esters, alcohols, and aromatic hydrocarbons. Insoluble in aliphatic hydrocarbons.; Stability:Stable to hydrolysis in the dark, DT50 >2210 d ( pH 5), 3740 d ( pH 7), 1370 d ( pH 9). Thermostable up to 300 °C.;
-
ToxicologyOral:Acute oral LD50 for rats >2000 mg/ kg. Percutaneous:Acute percutaneous LD50 for rats >5000 mg/kg. Non-irritating to eyes and skin ( tech.); severe irritant to eyes and skin (EC formulation) (rabbits). Inhalation: LC50 (4 h) for rats >2.1 mg/l air (for tech.). ADI:0.005 mg/ kg.
-
Environmental ProfileEcotoxicology:
Bees: LC50 (96 h, dust exposure) >0.29 mg/bee.Birds:Dietary LC50 (8 d) for bobwhite quail 4050, mallard ducks 2110 mg/ kg diet. LC50 (21 d) for bobwhite quail 2150 mg/ kg daily.Fish: LC50 (96 h) for bluegill sunfish 0.68 mg/l ( tech.).
Environmental fate:
Soil:Soil adsorption Koc 2100-9000 (clay, loam, sand, sandy loam, silty clay loam).Plant:In peanuts, degraded by three routes: oxidation at the benzylic carbon, leading to oxidative degradation products including a ketone and some lactones, substitution on the carbon next to the triazole ring, leading to triazolylalanine and triazolylacetic a -
Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)
Porduct NewsMore
Orthosulfamuron boosts sugarcane production, study finds
Glyphosate price plummets 40% in one year in Argentina
Indian govt stops imports of herbicide Glufosinate priced below Rs 1,289 per kg
Carbendazim fungicide wins victory in Brazilian Parliament
Corteva presents new pre-emergent herbicide Linear for sugarcane in Brazil
Picloram Triclopyr Aminopyralid
Revolutionizing disease prevention: BASF launches new rice fungicide Cevya® in China
Thiamethoxam is allowed again in Brazil by a judicial decision
Bayer develops alternative to glyphosate herbicide

 0
0 Subscribe
Subscribe