-
Common NameEsfenvalerate
-
中文通用名S-氰戊菊酯
-
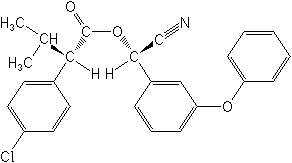
IUPAC(S)-α-cyano-3-phenoxybenzyl (S)-2-(4-chlorophenyl)-3-methylbutyrate
-
CAS[S-(R*,R*)]-cyano(3-phenoxyphenyl)methyl 4-chloro-2-(1-methylethyl)benzeneacetate
-
CAS No.66230-04-4
-
Molecular FormulaC25H22ClNO3
-
Molecular Structure
-
Category
-
ActivityEsfenvalerate is a synthetic pyrethroid insecticide which is used on a wide range of pests such as moths, flies, beetles, and other insects. It is used on vegetable crops, tree fruit, and nut crops. It may be mixed with a wide variety of other types of pesticides such as carbamate compounds or organophosphates.
-
FormulationDP = Dustable powder
-
PremixImidacloprid+esfenvalerate
Esfenvalerate+abamectin
Endosulfan+esfenvalerate
Malathion+esfenvalerate
Esfenvalerate+phoxim
Esfenvalerate+fenitrothion
-
Physical PropertiesMolecular weight:419.9; Physical form:Colourless crystals; ( tech., yellow-brown viscous liquid or solid at 23 °C). Density:1.26 (4-26 °C); Composition:Tech. is ×98% total isomers and ×75% resolved (S,S)- isomers. Melting point:59.0-60.2 °C; ( tech., 43.3-54 °C); Flash point:256 °C (Pensky-Martens); Vapour pressure:2 ×10-4 mPa (25 °C); Henry constant:4.20 × 10-2 Pa m3 mol-1 ( calc.); Partition coefficient(n-octanol and water):logP = 6.22 (25 °C); Solubility:In water 0.002 mg/l (25 °C). In xylene, acetone, chloroform, ethyl acetate, dimethylformamide, dimethyl sulfoxide >600, hexane 10-50, methanol 70-100 (all in g/ kg, 25 °C).; Stability:Relatively stable to heat and light. Stable to hydrolysis at < pH 5, 7 and 9 (25 °C).;
-
ToxicologyOral:Acute oral LD50 for rats 75-88 mg/ kg. Percutaneous:Acute percutaneous LD50 for rats >5000, rabbits >2000 mg/kg.
Slight skin irritant; mild eye irritant. Not a skin sensitiser. Phytotoxicity:Some injury has been noted on crucifers, cucumbers, aubergines, tomatoes, pears and mandarin oranges. -
Environmental ProfileEcotoxicology:
Bees: LD50 (contact) 0.017 mg/bee.Birds:Acute oral LD50 for bobwhite quail 381 mg/kg. LC50 (8 d) for bobwhite quail >5620, mallard ducks 5247 ppm.Daphnia: LC50 (48 h) 0.24 mg/l.Fish:Extremely toxic to aquatic animals. LC50 (96 h) for fathead minnows 0.690, bluegill sunfish 0.26, rainbow trout 0.26 g/l.
Environmental fate:
Animals:Rapid metabolism and elimination occurs in rats and other animals. Primary metabolism involves hydroxylation of 2'- and 4'- hydroxyl moieties, ester cleavage, hydroxylation and oxidation of the alcohol derivatives, oxidation of the cyano moiety and conjugSoil:In sand (0.38% o.m.), Kd (25 °C) 4.4; in sandy loam ( pH 7.3, 1.1% o.m.), Kd (25 °C) 6.4, DT50 88 d; in silty loam ( -
Transport InformationSignal Word:WARNING; Hazard Class:II(Moderately hazardous)
Porduct NewsMore
Sumitomo Chemical Acquires DuPont™ Asana® Insecticide Business
Related CompaniesMore
Jiangsu Greenscie Chemical Co., Ltd/Jiangsu Frey Agrochemicals Co., Ltd.
Country: China
Difenoconazole Dimethomorph Kresoxim-methyl Pyrimethanil Esfenvalerate Thiamethoxam Azoxystrobin+difenoconazole Tricyclazole Trifloxystrobin Pyraclostrobin Spiromesifen Trifloxystrobin+tebuconazole

 0
0 Subscribe
Subscribe